Partial Reprogramming Induced Rejuvenation (PRIR) by (OSK) via (AAIR)

(WikiLinks: iPSC) - (Last Revision: 02/28/2023)
(Reprogramming) - Last Revision: 10/07/2023

Introduction / Overview
◉ Reprogramed Induced Rejuvenation (RIR)
Glossary
Cellular identity: unique combination of molecular features (genomic, epigenetic, and transcriptomic) that leads to a specific phenotype and function in a cell.
Cellular plasticity: ability of a cell to change its identity under normal, pathological, or experimental contexts. Lineage infidelity: process by which a cell acquires a mixed identity comprising the coexistence of multiple transcriptional/ epigenetic programs.
Teratoma: benign tumors comprising differentiated derivatives from the three germ layers. Therefore, teratoma are indicative of the multilineage differentiation potential of a cell.
Transcription Factors: are switches that turn genes on or off. They are the primary tools for cellular reprogramming that can induce a state of youthfulness in cells, which is the fundamental premise behind RIR.
Reprogramming Induced Rejuvenation (RIR) is a revolutionary avenue of research that has demonstrated its ability to reverse biological aging by resetting epigenetic marks in cells to a more youthful state. Epigenetic marks are molecular tags that attach to DNA and histone proteins, influencing gene activity and playing a critical role in regulating the expression of longevity-associated genes. The selective activation of these genes results in the cellular, tissue or organismal systems resetting to a much younger age. This feat was originally accomplished on a cellular level by Shinya Yamanaka, in 2006, [✷ IP1], when his group Identified what are now universally referred to as Yamanaka factors. He published his findings in 2007 and received the 2012 Nobel prize in medicine for an advancement that will in all probability provide the first domino of a universally available, age-regression therapeutic. A complete description of inducing pluripotency via Yamanaka factors, can be found in the WikiLink above.
◉ Epigenetic Marks
Epigenetic Marks act as molecular tags on DNA and histone proteins directly controlling the expression of age-related genes. The strategic removal or alteration of these marks, reactivates younger gene expression patterns, leading to the rejuvenation of cellular, tissue, and organismal systems. Steve Horvath has conclusively proven that the expression of these epigenetic marks directly correlates with biological age. This provides both a critical validation marker for the determination of an organism's biological age and the impact each specific therapy exerts on biological age providing a valuable clinical marker.
◉ Transcription Factors
The expression of the pluripotency, transcription-factors: Oct4, Sox2, and Klf4 and c-Myc (OSKC) can revert somatic differentiated cells into pluripotent stem cells, in a process known as reprogramming. [✷ RIR6]. By limiting the reprogramming targets to only OSK, multiple parameters that improve safety are implemented.
◉ Interventional Meloculare Targets
Sections included below on this page describes in detail the potential targets addressable by AAIR agents and the classification of those agents. These interventional targets have been identified in both the RIR and iPSC literature, including work by Ocampo, Sinclair, Belmonte, Church, Melendez and many others.
These targets can be stratified by the molecular targets identified in the infographic on the right.
◉ Safety
The OSK method, targeting only the transcription factors Oct4, Sox2, and Klf4, and excluding the proto-oncogene c-Myc, offers multiple advantages over the initially utilized reprogramming quartet of OSKC. Eliminating C-myc removes the potential for generating teratomas, small disorganized tumor-like growths that have oncogenic potential due to their ability to form pluripotent cell masses. Driving the cellular lineage backwards to a younger age-state, using OSKC has the risk of losing the identity of the cell if this reprogramming is administered in a constant process (not pulsed) or for too long a period. Importantly, using the reduced set of transcription factors; O, S, and K, ensures cells don't lose their identity, permitting continuous treatment until desired anti-aging results is realized. Sinclairs group has determined that driving the OSK transcription factors either by small molecules indirectly activating these factors or using a cassette of transcripts that hit the promoters of these factors, results in the targets being driven backwards in their age lineages by approximately 80% and very importantly; no further! There is apparently a natural, inherent road block that prevents the continuation of the rejuvenation process to continue to the point where cellular identity is completely lost and pluripotency is obtained.
Small molecules have advantages because they can be cell permeable, non-immunogenic, more cost-effective, and can be more easily synthesized, preserved, and standardized. [✷ RIR0] The disadvantages are the very high cost and the long length of time safety testing and rigorous clinical trials require. The nutritional supplements identified herein, as effective interventions of the RIR molecular targets, have a long and well documented history of safety and efficacy in a wide variety of disease targets going back hundreds of years.
◉ Human Clinical Research
Multiple strategies have been developed by multiple research groups attempting to determine exactly what the optimal formulation of factors, doses, timing and treatment intervals should be to produce the most efficacious age-regression therapeutic regimen. To date this has not occurred in any human clinical studies. Dr. David Sinclair has just implemented the first primate study incorporating the cyclic expression of Yamanaka factors Oct4, Sox2 and Klf4, (OSK), delivered by Adeno-associated viruses (AAV) expression system, controlled by administration of doxycycline.[RIR19]
A minimal, select subset of 2 to 6 agents has been utilized in various models, cell types, and tissues, including mammals, to successfully and repeatedly rejuvenate these biological structures and systems. Notably, partial and reversible reprogramming does not change cell identity, but can reverse markers of aging in cells, improve the capacity of aged mice to repair tissue injuries, and extend longevity.
◉ Substatitutions
While some of the identified RIR agents should still be considered experimental, they all possess analogs or biosimilars that replicate their biological functions. Biosimilars are normally biological products that are highly similar to and have no clinically meaningful differences from an existing FDA-approved reference product. This first section identifies effective agents from ongoing research and provides an initial inventory of RIR targets to activate reprogramming. Each of these agent/targets fall into three major functional categories: epigenetics, cell signaling, and metabolic “switchers”. All these categories appear to be required in each small molecule (SM) cocktail to induce reprogramming. Remarkably, many enriched pathways of SM targets are related to aging, longevity, and age-related diseases, thus connecting them with cell reprogramming. The network analysis indicates that SM targets are highly interconnected and form protein-protein networks of a scale-free topology. (Fraifeld) All of the identified RIR agents that have been identified has a dedicated page below that completely describes the compound and its biological implications for reprogramming. Biological function, not molecular structure, is the primary goal of the identified substitutions shown on the right of each structure. It's essential to emphasize the distinction between biological function and molecular structure. Two molecules may have very similar structures, but vastly different functions, and vice versa. Therefore, focusing on the functional aspect increases, but does not guarantee the desired biological effect is achieved.
◉ AAIR
AAIR is a representative envelope of All Available Interventional Resources. This includes Amino Acids, Peptides, Proteins, Nutritional Supplements, Hormones and Drugs/Small Molecules. By intelligently applying these compounds through a phased and time-optimized introduction, we can harness their synergistic effects to optimize the PRIR process, with the gaols of achieving a highly impactful, age-regressive outcome.
◉ Clinical Goal
Eggan et al, observed that small molecules may functionally replace reprogramming transcription factors at either early or late stages of the process and that they can act by different mechanisms—by inducing the expression of the genes itself (Sox2 and c-Myc), a closely related family member, or an unrelated gene that can functionally rescue the omission of the reprogramming transcription factor. [T2]
The current strategie described on this page inspired from the work of Ocampo et al, [✷ RIR13] incorporating two agents; one targeting the monomer oxidase pathway (iMAO), and the other the transforming growth factor pathway (iTGF). Inhibiting these two pathway has a similar effect to inducing the three or four classic Yamanaka factors (Oct3/4, Sox2, c-Myc, Klf4). Ocampo’s group utilized a small molecule; Repsox (RepSox for its ability to replace Sox2 [RS1, RS2]) and an approved drug; Parnate,[P3] inhibiting MAO resulting in RIR. They established that 2c is sufficient to restore multiple aging phenotypes including genomic instability, epigenetic dysregulation, cellular senescence, and elevated reactive oxygen species. They also demonstrated life extension in an aging model; C. elegans.
Our goal is to replace the transcription factors and small molecules with AAIR substitutions having the same biological targets and mechanistic-pharmacological action. Nutritional supplements(NS) are often considered inferior to rationally designed drugs, but in this case, the two targets have multiple NS’s that have demonstrated potent and selective inhibition of the two primary targets Ocampo’s group has identified as being the minimally effective set. This drug like profile has been well characterized in peer reviewed literature for all of the NS substitutions we have identified. Coincidentally, many of the identified NS inhibitors of TGF beta-1/ALK5 are also inhibitors of the MAO pathway. This also includes GSK3 and histone deactylase, that are also positively correlated from the biological activity of the NS’s employed in this strategy. Multiple highly effective antioxidants also exist that have demonstrated an enhancing benefit on the RIR process. A real concern is the ease of which over modulation could be problematic utilizing the identified NS’s. This was demonstrated by Ocampo’s C. elegans studies where 200 uM produced a diminished lifespan responses compared to 50 and 100 uM doses. [✷ RIR13]
◉ ChatGPTv4 was incorporated as an Research and Organizational Tool
The organizational format of the description of each AIR agent was derived from a ChatGPT prompt extracting the relevant information for each compound and organized by specific categories defined by the indicated headings. Each RIR Interventional Agent is categorized by its AAIR and IEB classifications. ChatGPT has proven to be very valuable at making connections to an expanded library of AAIR agents that would have gone unexplored. That prompt is provided in full at the end of this page. All AI information was independently verified and those references provided.
◉ A great deal of information has been compiled on this page. This is a collaborative process and the information is likely to change. Archive the page is you want to refer to this specific information in the future.

Identified OSK-RIR-AAIR Agents
Effective initiation of organismal Reprogramming Induced Rejuvenation (RIR), utilizing Active Agents Inducing Rejuvenation (AAIR), requires the identification of agents that appropriately modulate the molecular pathways leading to the activation of crucial Transcription Factors that lead to rejuvenation. Given the wide range of targets that nutritional supplements can influence, it is equally vital to remove any agents that might stimulate pathways that work against or dampen those we aim to enhance. This extends to transcription factors. The success of RIR hinges on identifying AAIR agents that can act additively, complementarily, or synergistically to advance robust reprogramming and epigenetic remodeling. The spreadsheet provided serves as a tool to categorize AAIR (All Available Interventional Resources) candidates, streamlining their evaluation to maximize their rejuvenation potential.
Click [√] to Enlarge. For full access to the spreadsheet above please use the email link at the bottom of this page. The list of all 125 Klotho activating agents is also available for download at the bottom of this page.
Targeted AAIR elements inducing RIR
Identified Agents Substitutions MP Targets O S K Dosage MOA Notes
GSK3 inhibitor
CHIR99021
α-KG Alpha Ketoglutaric Acid
Vitamins
Retinoic Acid Vitamin A
Folic Acid, Vitamin B9
Cobalamin Vitamin B12
Ascorbic Acid, Vitamin C
Calciferol Vitamin D
Monomer Oxidase Inhibitor (MAO) see (LSD1 Inhibitors below)
Parnate
Lithium Lithium
[2022] Inhibition of glycogen synthase kinase 3 by lithium, a mechanism in search of specificity
[2012] Inhibition of GSK3 by lithium, from single molecules to signaling networks
AntiOxidants
NAC N-acetyl-cysteine Antioxidant
Histone Deactylase
Sodium Butyrate Sodium Butyrate MTI DNMT 25X
Minerals /Metals
Magnesium
Zinc
LSD1 Inhibitors
Approved Drugs:
Parnate, TCP
Small Molecules:
BIX-01294
Nutrational Supplements
Lysine-specific demethylase 1 (LSD1) is an enzyme that demethylates histone proteins, specifically demethylating mono- and di-methylated lysine 4 on histone H3 (H3K4me1/2), leading to transcriptional repression. Inhibiting LSD1 can lead to the accumulation of H3K4me1/2, thereby promoting transcriptional activation.
Connection to Transcription Factors O, S, and K:
Oct4
Oct4 is a critical transcription factor involved in maintaining the pluripotency of embryonic stem cells.
LSD1 Inhibition: Inhibition of LSD1 can lead to increased levels of H3K4me1/2 at the promoters of genes regulated by Oct4, thereby enhancing their transcription. This process is essential for maintaining the self-renewal and pluripotent state of stem cells.
Sox2
Sox2 is another key transcription factor required for maintaining pluripotency and self-renewal in stem cells.
LSD1 Inhibition: Similar to Oct4, Sox2-regulated genes can also be activated by the increased H3K4me1/2 levels resulting from LSD1 inhibition. This epigenetic change supports the transcriptional programs necessary for stem cell maintenance and pluripotency.
Klf4
Klf4 is a transcription factor involved in the regulation of cell proliferation, differentiation, and pluripotency.
LSD1 inhibition can enhance the transcription of Klf4 target genes by increasing H3K4me1/2 marks at their promoters. This upregulation supports the expression of genes that are crucial for maintaining the undifferentiated state of stem cells.
Mechanism of Action:
Histone Modification: LSD1 inhibition prevents the removal of methyl groups from H3K4me1/2, leading to the accumulation of these marks.
Chromatin State: Increased H3K4me1/2 is associated with a more open chromatin state, which facilitates the binding of transcription factors and the recruitment of the transcriptional machinery.
Gene Activation: The open chromatin state and enhanced transcription factor binding promote the transcription of genes involved in maintaining pluripotency and self-renewal.
Implications for Stem Cell Biology and Reprogramming:
Pluripotency Maintenance: By promoting the activation of Oct4, Sox2, and Klf4 target genes, LSD1 inhibitors can help maintain the pluripotent state of stem cells.
Cell Reprogramming: LSD1 inhibition can be utilized in cellular reprogramming protocols to enhance the efficiency of converting somatic cells into induced pluripotent stem cells (iPSCs) by activating the core pluripotency network governed by Oct4, Sox2, and Klf4.
Potential Applications:
Regenerative Medicine: Enhancing the activation of pluripotency genes through LSD1 inhibition could improve the generation and maintenance of iPSCs for regenerative therapies.
Cancer Research: Since LSD1 is often overexpressed in various cancers, its inhibition could be explored as a therapeutic strategy to reactivate tumor suppressor genes and inhibit cancer cell proliferation.
In summary, LSD1 inhibition facilitates the activation of transcription factors Oct4, Sox2, and Klf4 by increasing H3K4me1/2 marks, leading to enhanced transcription of genes crucial for maintaining stem cell pluripotency and self-renewal. This mechanism has significant implications for stem cell biology, cellular reprogramming, and potential therapeutic applications.
TGFβ/ALK5 inhibitor
Approved Drugs
Small Molecules
RepSox TGF1b/ALK5 Sox2
Nutrational Supplements
Curcumin TGF1b/ALK5. Sox2
EGCG TGF1b/ALK5. Sox2
Genistein TGF1b/ALK5 Sox2
Resveratrol TGF1b/ALK5 Sox2
Quercetin TGF1b/ALK5 Sox2
[2017] Molecular docking analysis of curcumin analogues against kinase domain of ALK5
Transforming growth factor-beta 1 (TGF-β1) is a multifunctional cytokine that plays a critical role in cell growth, differentiation, apoptosis, and cellular homeostasis. It is particularly known for its role in inducing epithelial-to-mesenchymal transition (EMT), promoting fibrosis, and inhibiting the proliferation of certain cell types, including stem cells.
Connection to Transcription Factors O, S, and K:
Oct4
Oct4 is essential for maintaining the pluripotency and self-renewal of embryonic stem cells.
TGF-β1 Inhibition: TGF-β1 signaling typically represses the expression of pluripotency factors like Oct4. Inhibition of TGF-β1 can relieve this repression, leading to increased expression of Oct4 and the maintenance of the pluripotent state in stem cells.
Sox2
Sox2, along with Oct4, is a key transcription factor required for pluripotency and the self-renewal of stem cells.
TGF-β1 Inhibition: Inhibition of TGF-β1 can lead to an upregulation of Sox2, as TGF-β1 signaling pathways often negatively regulate the expression of pluripotency genes. Blocking TGF-β1 signaling promotes an environment conducive to maintaining stem cell characteristics and pluripotency.
Klf4
Klf4 is involved in regulating cell proliferation, differentiation, and maintaining the pluripotent state of stem cells.
TGF-β1 Inhibition: Klf4 expression can be suppressed by TGF-β1 signaling. Inhibition of TGF-β1 leads to the activation of Klf4, contributing to the maintenance and induction of pluripotency in stem cells.
Mechanism of Action:
Signal Pathway Interference: TGF-β1 signals through the Smad pathway and other non-Smad pathways to exert its effects on gene expression. Inhibition of TGF-β1 prevents the phosphorylation and activation of Smad proteins, which are responsible for transmitting the TGF-β1 signal to the nucleus to repress pluripotency genes.
Chromatin State: TGF-β1 inhibition can lead to a more open chromatin state around the promoters of pluripotency genes, facilitating the binding of Oct4, Sox2, and Klf4 and enhancing their transcriptional activity.
Gene Activation: By blocking the repressive effects of TGF-β1 signaling, inhibition allows for the activation of genes regulated by Oct4, Sox2, and Klf4, promoting pluripotency and self-renewal.
Implications for Stem Cell Biology and Reprogramming:
Pluripotency Maintenance: Inhibiting TGF-β1 helps maintain the expression of Oct4, Sox2, and Klf4, which are essential for keeping stem cells in an undifferentiated state.
Cell Reprogramming: TGF-β1 inhibition is a crucial step in reprogramming somatic cells into induced pluripotent stem cells (iPSCs). By preventing TGF-β1-mediated repression, the activation of Oct4, Sox2, and Klf4 is facilitated, enhancing the efficiency of reprogramming.
Potential Applications:
Regenerative Medicine: TGF-β1 inhibitors can be used to maintain and expand pluripotent stem cells for regenerative therapies by ensuring the sustained activation of Oct4, Sox2, and Klf4.
Cancer Research: Since TGF-β1 signaling can contribute to tumor progression and metastasis through EMT, inhibiting TGF-β1 could have therapeutic potential in treating cancers by reversing EMT and reactivating tumor suppressor genes.
In summary, TGF-β1 inhibition promotes the activation of transcription factors Oct4, Sox2, and Klf4 by relieving the repressive effects of TGF-β1 signaling. This results in the upregulation of genes essential for maintaining pluripotency and self-renewal in stem cells, with significant implications for stem cell biology, cellular reprogramming, and potential therapeutic applications.Parnate, TCP
Site Navigational Note: Ref links that contain a ⟦✷⟧
are key documents supporting this concept and are highlighted in the references.
Paragraphs beginning with “⫸” and ending with “⫷,” indicate that the references noted within the paragraph will be found within the original cited article. ⫷⟦X⟧
The cyclic/pulsed transient dosing regimen incorporated herein is derived from the Belmonte mouse studies. [✷ RIR4] His brief description of the process was: “Cellular reprogramming by transient expression of Yamanaka factors ameliorates age-associated symptoms, prolongs lifespan in progeroid mice, and improves tissue homeostasis in older mice.
His group demonstrated that by pulsing (via doxycycline induction of a Yamanak cassette of genetic signals targeting an OSKM polycystronic cassette (4F), that RIR could be successfully achieved.
The fact that reprogramming proceeds in a stepwise manner allows for the induction of partial reprogramming without the complete loss of cellular identity by short exposure to the Yamanaka factors [RIR1]. Based on Ocampo’s work the same process should apply to small molecules and/or nutritional supplements.
Safety and Timing
The image on the right demonstrates that by eliminating the activation of C-Myc in the reprogramming process two benefits are achieved. 1) the likelihood of losing cell identity is decreased and 2) The risk of promoting oncogenic processes including the induction of teratomas is decreased. Image sources: Live Bioscience.
It is also important to note that the beneficial effects of induced reprogramming are conferred prior to the loss of cell identity. “Importantly, these data demonstrate that the process of cellular rejuvenation (transcriptomic, epigenetic, and cellular levels,) is engaged very early, rapidly, and broadly in the reprogramming process. These transcriptional changes occur before any epigenetic reprogramming of cellular identity takes place, a novel finding in the field.” [✷ RIR6] It has also been demonstrated [✷ RIR4] that tumor formation and loss of cellular identity can be prevented by short term administration or pulsed induction of transcription factors via Yamanaka factors or by triggering downstream activation by incorporating small molecules and/or nutritional supplements.
These two pathways in all probability, provide for a slightly slower, less robust, rejuvenation process when incorporating three as opposed to four Yamanaka nuclear factors, but may provide an increased level of safety while still improving; immunity, health-span, quality of life, and simultaneously preventing/resolving diseases.
Effectiveness of Nutritional Supplements (NS)
Click [√] to Enlarge. Source: Epigallocatechin gallate (EGCG) suppresses epithelial-Mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF- β 1/Smad signaling pathways
EGCG provides an excellent example of how effective the selected supplements can be at replacing/duplicating the drug inducing activities of the active agents incorporated by the Ocampo group. It’s effectiveness in modulating molecular targets in cancer are detailed below.
Click [√] to Enlarge
⫸ Epigallocatechin-3-gallate (EGCG) has emerged as a distinguished chemopreventive product because of its ability to regulate a myriad of oncogenic signaling pathways. Based on its scientifically approved anticancer activity and encouraging results obtained from preclinical trials, it is also being tested in various phases of clinical trials. A series of clinical trials associated with green tea extracts and EGCG are providing clues about significant potential of EGCG to mechanistically modulate wide ranging signal transduction cascades. In this review, we comprehensively analyzed regulation of JAK/STAT, Wnt/ β -catenin, TGF/SMAD, SHH/GLI, NOTCH pathways by EGCG. We also discussed most recent evidence related to the ability of EGCG to modulate non-coding RNAs in different cancers. Methylation of the genome is also a widely studied mechanism and EGCG has been shown to modulate DNA methyltransferases (DNMTs) and protein enhancer of zeste-2 (EZH2) in multiple cancers. ⫷ [E6] EGCG was noted to completely block the phosphorylation of SMAD2/3 and nuclear accumulation of SMAD4. [R3]
◉ Targets and Stratifications of RIR Agents
◉ AAIR constitutes “All Available Interventional Resources,” as Classified Below.
AIR is a representative envelope of all Available Interventional Resources. This includes Amino Acids, Peptides, Proteins, Nutritional Supplements, Hormones and Drugs/Small Molecules. Combined into a rational treatment strategy, this potent combination of available resources provides all of us with a highly impactful, age-regressive armamentarium.
Ocampo's group has demonstrated that a combination of two small molecules, referred to as "inducers," can ameliorate aging phenotypes, including cellular senescence and oxidative stress, in the nematode Caenorhabditis elegans and mice. [✷ RIR13] This partial chemical reprogramming combination can improve genomic instability and epigenetic alterations in aged human cells, suggesting that it is possible to extend lifespan and improve key drivers of aging via chemical-induced partial reprogramming. The molecular targets of these two compounds are addressable by AIR agents, which are identified in the following sections utilizing the categories identified in the graphic on the left, to safely activate the RIR protocol.
◉ Yamanaka Transcriptional Factors (TF); Molecular Activation Pathways (MAP); and AIR Agent Activators / Inhibitors
⬆
⬇
Targeting of three Yamanaka factors are employed in the stratgie described here. A more complete description of Yamanaka Factors can be found here. The three TFs are: OCT4, SOX2 and KLF4.
SOX2 [⬆]
SOX2 is a transcription factor, a protein that binds to DNA and regulates the expression of genes, which plays a crucial role in embryonic development and the maintenance of adult stem cells.
REPSOX [⬆]
Repsox is a small molecule inhibitor that has been shown to enhance the expression of SOX2, a transcription factor that plays a critical role in the maintenance of pluripotency and self-renewal in embryonic stem cells. Repsox is known to inhibit the activity of the enzyme glycogen synthase kinase 3 beta (GSK-3β), which is involved in the Wnt signaling pathway.
The Wnt signaling pathway is a complex network of molecular interactions that regulate various cellular processes, including proliferation, differentiation, and stem cell self-renewal. GSK-3β is a negative regulator of the Wnt pathway, and its inhibition can lead to the stabilization and accumulation of β-catenin, a key transcriptional co-activator that plays a critical role in the activation of Wnt target genes.
Recent studies have shown that Repsox-mediated inhibition of GSK-3β leads to the upregulation of SOX2 through the activation of the Wnt pathway. The precise mechanism by which this occurs is not fully understood, but it is thought to involve the activation of downstream Wnt target genes that regulate the expression and stability of SOX2 mRNA and protein.
In summary, Repsox interfaces with the Wnt signaling pathway by inhibiting GSK-3β, which leads to the upregulation of SOX2 through the activation of downstream Wnt target genes.
WNT [⬆]
One of the key pathways that activate SOX2 expression is the Wnt signaling pathway. Wnt ligands bind to Frizzled receptors, leading to the activation of β-catenin, which translocates to the nucleus and binds to the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors. This complex then activates the transcription of SOX2, among other target genes.
AIR Activators of the WNT Signaling Pathway:
Curcumin: Curcumin is a polyphenol found in turmeric that has been shown to activate the Wnt signaling pathway. Studies have shown that curcumin can activate β-catenin and increase the expression of Wnt target genes.
Epigallocatechin gallate's (EGCG): EGCG is a polyphenol found in green tea that has been shown to activate the Wnt signaling pathway. Studies have shown that EGCG can increase the expression of Wnt target genes and activate β-catenin.
Resveratrol: Resveratrol is a polyphenol found in grapes, berries, and peanuts that has been shown to activate the Wnt signaling pathway. Studies have shown that resveratrol can activate β-catenin and increase the expression of Wnt target genes.
Berbrine: Berberine is a natural compound found in several plants that has been shown to activate the Wnt signaling pathway. Studies have shown that berberine can increase the expression of Wnt target genes and activate β-catenin.
Astragalus membranac Astragalus membranaceus: Astragalus membranaceus is a medicinal herb commonly used in traditional Chinese medicine. Studies have shown that extracts of this herb can activate the Wnt signaling pathway and increase the expression of Wnt target genes.
TGF-Beta-1 [⬇]
TGF-Beta-1 has been shown to inhibit SOX2 expression. TGF-beta1 is a cytokine that plays a critical role in many cellular processes, including cell differentiation and development. Studies have shown that TGF-beta1 treatment can decrease the expression of SOX2 in various cell types, including embryonic stem cells and cancer cells.
The mechanism by which TGF-beta1 inhibits SOX2 expression is not fully understood, but it is thought to involve the activation of Smad proteins. TGF-beta1 binds to its receptor, leading to the activation of Smad2 and Smad3. These Smad proteins then form a complex with Smad4 and translocate to the nucleus, where they can bind to the SOX2 promoter region and inhibit its expression.
Overall, TGF-beta1 is a potent inhibitor of SOX2 expression and plays an important role in regulating cell fate decisions and differentiation.
AIR Inhibitors of TGF-Beta-1:
Curcumin, for example, is a polyphenol found in turmeric that has anti-inflammatory and antioxidant properties. Studies have shown that curcumin can inhibit TGF-beta1-induced signaling in various cell types, including fibroblasts and cancer cells.
Resveratrol, another polyphenol found in red grapes and berries, has been shown to inhibit TGF-beta1-induced signaling in lung fibroblasts and skin cells.
EGCG, a polyphenol found in green tea, has been shown to inhibit TGF-beta1-induced signaling in various cell types, including lung fibroblasts and prostate cancer cells.
Quercetin , a flavonoid found in many fruits and vegetables, has been shown to inhibit TGF-beta1-induced signaling in lung fibroblasts and breast cancer cells.
FGF (fibroblast growth factor) Signaling Pathway [⬆]
Another pathway that activates SOX2 is the FGF signaling pathway. FGF ligands bind to their receptors, leading to the activation of the Ras/MAPK pathway and subsequent phosphorylation of the transcription factor Ets1. Ets1 then binds to the SOX2 promoter region, resulting in its activation.
AIR Activators of FGF (fibroblast growth factor):
Omega-3 fatty acids: Omega-3 fatty acids are essential fatty acids found in fish, flaxseed, and walnuts. Studies have shown that omega-3 fatty acids can activate the FGF signaling pathway and promote angiogenesis (the formation of new blood vessels).
Quercetin: Quercetin is a flavonoid found in several plants, including onions, apples, and berries. Studies have shown that quercetin can activate the FGF signaling pathway and promote angiogenesis.
Genistein: Genistein is an isoflavone found in soybeans and other legumes. Studies have shown that genistein can activate the FGF signaling pathway and promote angiogenesis.
Resveratrol: Resveratrol is a polyphenol found in grapes, berries, and peanuts. Studies have shown that resveratrol can activate the FGF signaling pathway and promote angiogenesis.
Vitamin E: Vitamin E is a fat-soluble vitamin found in several foods, including nuts, seeds, and leafy greens. Studies have shown that vitamin E can activate the FGF signaling pathway and promote angiogenesis.
Vitamin D: Vitamin D: Vitamin D is a fat-soluble vitamin found in several foods, including fatty fish, egg yolks, and fortified dairy products. Studies have shown that vitamin D can activate the FGF signaling pathway and promote angiogenesis.
Berberine: Berberine is a natural compound found in several plants, including barberry and goldenseal. Studies have shown that berberine can activate the FGF signaling pathway and promote angiogenesis.
Curcumin: Curcumin is a polyphenol found in turmeric. Studies have shown that curcumin can activate the FGF signaling pathway and promote angiogenesis.
Epigallocatechin-3-gallate (EGCG): EGCG is a polyphenol found in green tea. Studies have shown that EGCG can activate the FGF signaling pathway and promote angiogenesis.
Ginkgo biloba: Ginkgo biloba is an herbal supplement commonly used for cognitive enhancement. Studies have shown that ginkgo biloba can activate the FGF signaling pathway and promote angiogenesis.
BMP (Bone Morphogenetic Protein) [⬇]
SOX2 can also be inhibited by the BMP signaling pathway. BMP ligands bind to their receptors, leading to the activation of Smad proteins, which then translocate to the nucleus and bind to the SOX2 promoter, resulting in its inhibition.
AIR Activators of FGF (fibroblast growth factor):
Noggin: Noggin is a protein that inhibits BMP signaling by binding to BMP ligands and preventing them from binding to their receptors. Noggin is found naturally in the body, but it can also be taken as a supplement.
Chrysin: Chrysin is a flavonoid found in several plants, including passionflower and honey. Studies have shown that chrysin can block BMP signaling by inhibiting the phosphorylation of Smad proteins, which are downstream targets of the BMP signaling pathway.
Quercetin: Quercetin is a flavonoid found in several plants, including onions, apples, and berries. Studies have shown that quercetin can block BMP signaling by inhibiting the phosphorylation of Smad proteins.
Genistein: Genistein is an isoflavone found in soybeans and other legumes. Studies have shown that genistein can block BMP signaling by inhibiting the phosphorylation of Smad proteins.
Epigallocatechin-3-gallate (EGCG): EGCG is a polyphenol found in green tea. Studies have shown that EGCG can block BMP signaling by inhibiting the phosphorylation of Smad proteins.
Epigenetic Modifications: DNA Methylation [⬇]; Histone Acetylation [⬆]
In addition, SOX2 expression can be regulated by epigenetic modifications such as DNA methylation and histone acetylation. DNA methylation of the SOX2 promoter region can lead to its silencing, while histone acetylation can promote its activation.
Overall, the activation or inhibition of SOX2 expression is a complex process that involves multiple signaling pathways and epigenetic modifications.
It is not desirable to up-regulate or activate DNA methylation of the SOX2 promoter region since DNA methylation typically leads to gene silencing. However, there are several nutritional supplements that have been shown to inhibit DNA methylation, which could potentially lead to increased expression of SOX2.
AIR Inhibitors of DNA Methylation:
Folic acid: Folic acid is a B vitamin found in leafy greens, citrus fruits, and fortified grains. Studies have shown that folic acid can inhibit DNA methylation and increase the expression of certain genes.
Vitamin B12: Vitamin B12 is a B vitamin found in animal products, including meat, fish, and dairy. Studies have shown that vitamin B12 can inhibit DNA methylation and increase the expression of certain genes.
S-adenosylmethionine (SAMe): SAMe is a naturally occurring compound found in the body that plays a role in methylation reactions. Studies have shown that SAMe can inhibit DNA methylation and increase the expression of certain genes.
Curcumin: Curcumin is a polyphenol found in turmeric. Studies have shown that curcumin can inhibit DNA methylation and increase the expression of certain genes.
EGCG: EGCG is a polyphenol found in green tea. Studies have shown that EGCG can inhibit DNA methylation and increase the expression of certain genes.
Methyl-donors: Methyl-donors are compounds that provide methyl groups for DNA methylation reactions. Nutritional supplements such as betaine, choline, and methionine are examples of methyl-donors and should be avoided if the goal is to prevent DNA methylation.
Alcohol: Chronic alcohol consumption has been shown to induce DNA hypermethylation in various tissues, including the liver and brain. Therefore, it is advisable to limit alcohol consumption to prevent unwanted DNA methylation.
Overall, these nutritional supplements have shown potential in inhibiting DNA methylation, which could potentially lead to increased expression of SOX2. However, more research is needed to determine their effectiveness and safety as nutritional supplements. It is also important to note that excessive inhibition of DNA methylation can have negative effects on cell growth and development, so these supplements should be used with caution and under the guidance of a healthcare professional.
AAIR Activation of Histone Acetylation:
There are several nutritional supplements that have been shown to upregulate or activate histone acetylation. These supplements include:
Resveratrol: Resveratrol is a polyphenol found in grapes, berries, and peanuts. Studies have shown that resveratrol can increase histone acetylation by activating the SIRT1 deacetylAcase enzyme.
Curcumin: Curcumin is a polyphenol found in turmeric. Studies have shown that curcumin can increase histone acetylation by inhibiting the activity of HDAC enzymes.
Sulforaphane: Sulforaphane is a compound found in cruciferous vegetables, including broccoli and cauliflower. Studies have shown that sulforaphane can increase histone acetylation by inhibiting the activity of HDAC enzymes.
Butyrate: Butyrate is a short-chain fatty acid produced by gut bacteria during the fermentation of dietary fiber. Studies have shown that butyrate can increase histone acetylation by inhibiting the activity of HDAC enzymes.
Vitamin D: Vitamin D is a fat-soluble vitamin found in several foods, including fatty fish, egg yolks, and fortified dairy products. Studies have shown that vitamin D can increase histone acetylation by regulating the expression of genes involved in histone acetylation.
Overall, these nutritional supplements have shown potential in upregulating or activating histone acetylation, which plays an important role in regulating gene expression, cell growth, and differentiation. However, more research is needed to determine their effectiveness and safety as nutritional supplements. It is also important to note that excessive activation of histone acetylation can have negative effects on cell growth and development, so these supplements should be used with caution and under the guidance of a healthcare professional.
◉ AAIR IEB RIR
All AAIR-RIR’s, are described in the next section. These interventional targets have been identified in both the RIR and iPSC literature, including work by Ocampo, Sinclair, Belmonte, Church, Melendez and many others. Multiple agents have been utilized to address those targets in vitro and in small animal models utilizing AIR supplemental substitutions that can replicate the pharmacological activity of transcription factor activators, or small molecule activators, identified as reprogramming molecules.
The successful use of these factors suggests that activation of the Wnt signaling pathway, inhibition of TGF-β signaling, and release of histones are important in the induction process for rejuvenation.
◉ All Agents Facilitating the Reprogramming Process Fall into Three Categories: Induce; Enhance or Blocking the RIR process.
They directly “induce,” rejuvenation by activating the transcription factors that activate Yamanaka Factors and/or molecular pathways that accomplish the same goal.
They “Enhance,” the process by concomitantly or synergistically facilitating the action of inducing agents or changing the cellular environment to improve the response of inducers.
Blocking agents, “Block,” or counteract specific RIR biological processes that would otherwise have resulted in successful RIR.
Taken together, the data show that small-molecule-mediated reprogramming of all germline lineages into SmiPSCs appears to be possible, suggesting a new way of understanding pluripotency. [RIR5]
◉ Administration of RIR Agents must be strategized by each of the fellowing eight (8) considerations.
Reprogramming cells is similar to cracking a safe; you must determine the right combination, sequence, duration and interval.
Administration of RIR Agent (NDIA)
Remove all blocking Agents from diet
Dosage
Time of Day
Duration (Number of Hours to maintain target dosage)
Interval (Schedule for repeating the dosage and duration.)
Total Window of Exposure (Number of treatment cycles)
Break (Period between last and next treatment cycle)
The SM targets fall into three major functional categories: Enhancers/Inducers/
• Epigenetics, [Supplementary Table 3] >
The most SMs inhibit either methyltransferases (HMTs and DNMTs, 9 and 6, respectively) or HDACs (n = 4). Other molecules possess either dual activity (HDAC inducers and/or inhibitors, n = 3) or combined (inhibition of HMT+DNMT or DNMT+HDAC) activities. This, respectively, shifts the condensed form of chromatin (heterochromatin) towards a relaxed state (euchromatin) or decreases the level of DNA methylation, thereby ensuring more DNA to be available for transcription.
• Cell Signaling, and [Supplementary Table 2] >
The most frequently used in SM cocktails) signaling modifiers include inhibitors of TGFβ and Hedgehog signaling, both involved in cell differentiation [15, 16].
• Metabolic “Switchers”. [Supplementary Table 4] >
Lastly, metabolic modifiers switch the metabolism from oxidative phosphorylation towards glycolysis, mostly through the inhibition of the GSK3 enzyme [17].
Other SMs (n = 8; 8.7%) include antioxidants, regulators of calcium transport, autophagy, etc. [Supplementary Table 5]
All of these categories appear to be required in each SM cocktail to induce cell reprogramming. Remarkably, many enriched pathways of SM targets are related to aging, longevity, and age-related diseases, thus connecting them with cell reprogramming. The network analysis indicates that SM targets are highly interconnected and form protein-protein networks of a scale-free topology. The extremely high contribution of hubs to network connectivity suggests that (i) cell reprogramming may require SM targets to act cooperatively, and (ii) their network organization might ensure robustness by resistance to random failures.
Vadim E. Fraifeld, et al, first compiled a full list of SMs established thus far, based on a keyword meta-analysis of the literature. Comprehensive data mining with subsequent curation (see Methods) resulted in a total of 92 chemical compounds (Supplementary Table 1) that can either induce or enhance pluripotency, alone or in combination with TFs. These compounds for chemical reprogramming were named “Small Molecules” (SMs) because of their relatively low molecular weight [9], which ranges from 42.4 g/mol (LiCl) to 914.2 g/mol (Rapamycin). The vast majority of SMs represent organic compounds belonging to various chemical classes; however, among SMs were also several inorganic compounds (e.g., Lithium salts).
[2021] Small molecules for cell reprogramming: a systems biology analysis
[2013] Roles of small molecules in somatic cell reprogramming
Interventional Molecular Targets
RIR targets are described in the next section. These interventional targets have been identified in both the RIR and iPSC literature, including work by Ocampo, Sinclair, Belmonte, Church, Melendez and many others.
These targets can be stratified by the molecular targets identified in the infographic on the right.
◉ Upper Level Categories of RIR Molecular Pathways
◉ Chromatin Remodeling
Chromatin remodeling is a complex process that involves the modification of the chromatin structure, which can either activate or silence gene expression. Reprogramming of cells requires the activation of specific genes and the silencing of others, and as such, it is dependent on chromatin remodeling.
The compounds that induce chromatin remodeling and reprogramming of cells include
1) histone deacetylase (HDAC) inhibitors,
2) DNA methyltransferase (DNMT) inhibitors, and
3) histone methyltransferase (HMT) inhibitors.
HDAC inhibitors such as trichostatin A (TSA) and valproic acid (VPA) prevent the removal of acetyl groups from histones, leading to a more relaxed chromatin structure and increased gene expression. DNMT inhibitors such as 5-azacytidine and 5-aza-2'-deoxycytidine prevent DNA methylation, which is a common mechanism for gene silencing. HMT inhibitors such as chaetocin and BIX-01294 prevent the addition of methyl groups to histones, which can also lead to gene silencing.
Together, these compounds induce changes in the chromatin structure that allow for the activation of genes that are necessary for reprogramming of cells.
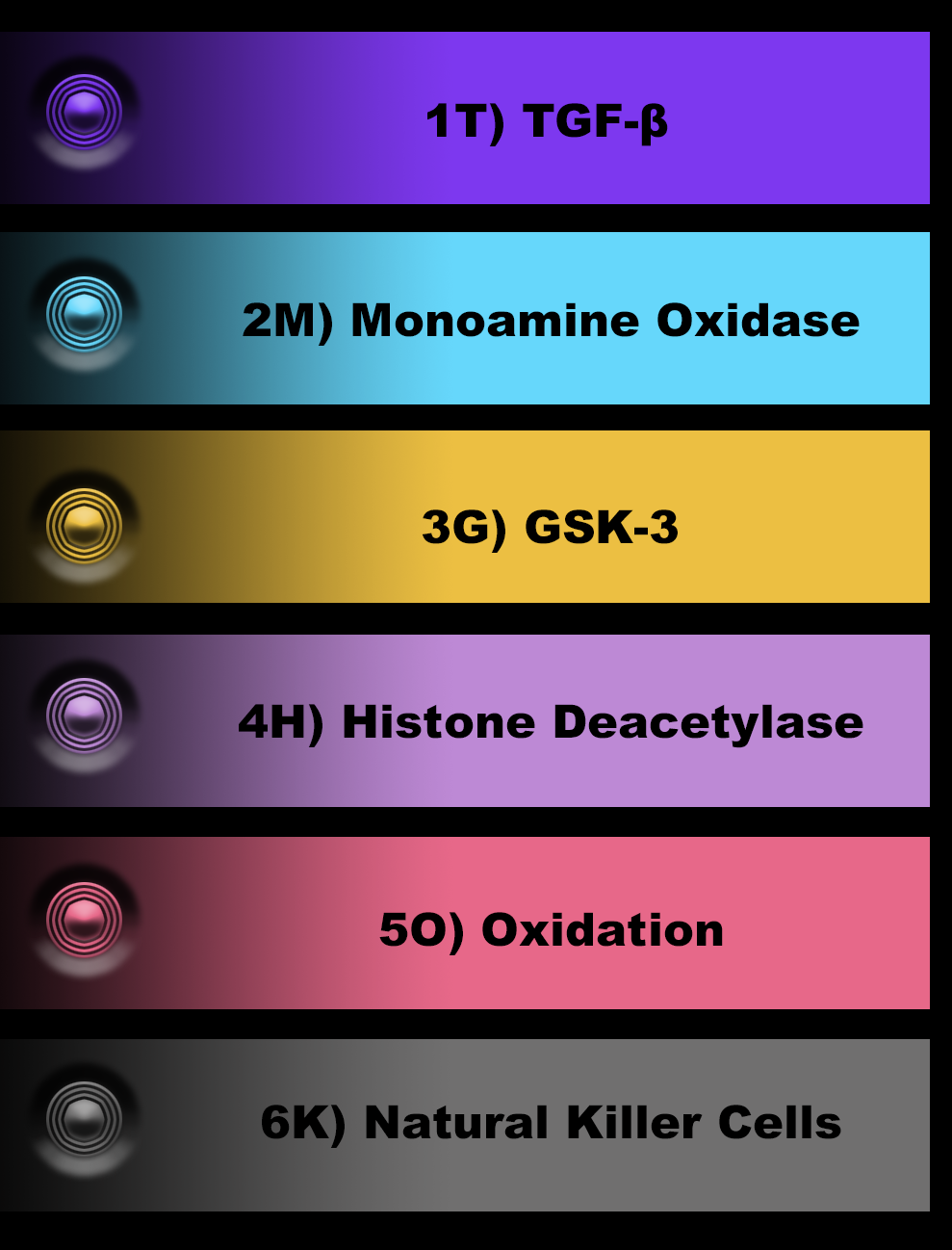
◉ (1T) / TGF-β1 /
Transforming Growth Factor beta /
The TGF-beta-1 pathway is a signaling pathway that plays a crucial role in many biological processes, including cell growth, differentiation, apoptosis, and immune regulation. The TGF-beta-1 pathway has been shown to be involved in cellular aging and senescence, and its dysregulation has been implicated in age-related diseases such as cancer and fibrosis.
In relation to the three questions:
The TGF-beta-1 pathway is involved in epigenetic regulation, specifically DNA methylation. TGF-beta-1 has been shown to regulate DNA methyltransferase activity, leading to changes in DNA methylation patterns. Therefore, the TGF-beta-1 pathway may play a role in the epigenetic reprogramming pathway involved in RIR.
The TGF-beta-1 pathway is typically upregulated in senescent cells and has been implicated in the promotion of cellular senescence. Therefore, to achieve RIR, the TGF-beta-1 pathway may need to be downregulated.
The TGF-beta-1 pathway is involved in the regulation of other pathways, including the p53 pathway and the PI3K/Akt pathway, which are important in cellular aging and senescence. The TGF-beta-1 pathway can activate the p53 pathway, leading to cell cycle arrest and apoptosis, and can also inhibit the PI3K/Akt pathway, leading to decreased cell proliferation and increased cellular senescence. The fine-tuning of these pathways is crucial for achieving RIR, and the TGF-beta-1 pathway may need to be carefully regulated to achieve rejuvenation.
Overall, the TGF-beta-1 pathway is a complex signaling pathway that is involved in many cellular processes, including aging and rejuvenation. Its regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand its role in cellular rejuvenation.
Programming Note: Here, we report the discovery of compounds that can replace the central reprogramming factor Sox2. We demonstrate that one of these chemicals specifically acts by inhibiting Tgf- β signaling. Interestingly, this compound does not act by inducing Sox2 expression in the target fibroblasts. Instead, we show that it enables reprogramming through the induction of Nanog transcription in a stable, partially reprogrammed cell type that accumulates in the absence of Sox2.
xxx
The MAO (Monoamine oxidase) pathway is involved in the metabolism of monoamine neurotransmitters, such as dopamine, serotonin, and norepinephrine. Dysregulation of the MAO pathway has been implicated in several psychiatric and neurodegenerative disorders, including depression, anxiety, and Parkinson's disease. Here's how the MAO pathway relates to the three questions:
The MAO pathway has been shown to be involved in epigenetic regulation. Specifically, MAO enzymes can modify histone methylation patterns, leading to changes in gene expression. Therefore, the MAO pathway may play a role in the epigenetic reprogramming pathway involved in RIR.
Inhibition of the MAO pathway has been shown to increase the levels of monoamine neurotransmitters, such as dopamine and serotonin, which can have positive effects on mood and cognitive function. Therefore, the MAO pathway may need to be downregulated to achieve RIR.
The MAO pathway is linked to other pathways involved in cellular aging and senescence, such as the oxidative stress pathway and the inflammatory pathway. The metabolism of monoamine neurotransmitters by the MAO pathway can generate reactive oxygen species, leading to oxidative stress and damage to cellular components. The MAO pathway can also activate inflammatory pathways through the production of pro-inflammatory cytokines. The regulation of the MAO pathway and its interaction with other pathways are crucial for achieving RIR and restoring cellular function in aged cells.
Overall, the MAO pathway is a complex signaling pathway that is involved in several biological processes, including aging and rejuvenation. Its regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand its role in cellular rejuvenation.
◉ (3G) / GSK-3 / Glycogen Synthase Kinase-3 / / [ Up-Regulate ]
The GSK-3 (Glycogen Synthase Kinase-3) pathway is a complex signaling pathway that plays a critical role in many biological processes, including cell growth, differentiation, apoptosis, and metabolism. Dysregulation of the GSK-3 pathway has been implicated in the development of many diseases, including cancer, Alzheimer's disease, diabetes, and bipolar disorder. Here's how the GSK-3 pathway relates to the three questions:
The GSK-3 pathway has been shown to regulate gene expression through the phosphorylation of transcription factors, such as beta-catenin and cAMP-response element-binding protein (CREB). Therefore, the GSK-3 pathway may play a role in the epigenetic reprogramming pathway involved in RIR. The GSK-3 molecular pathway is a complex signaling pathway that plays a critical role in many biological processes, including cell growth, differentiation, apoptosis, and metabolism. GSK-3 is a serine/threonine kinase that phosphorylates a wide variety of proteins, including glycogen synthase, tau, beta-catenin, and many others. GSK-3 is regulated by several upstream signaling pathways, including the PI3K/Akt, Wnt, and Hedgehog pathways, which can either activate or inhibit GSK-3 activity.
When GSK-3 is active, it phosphorylates downstream targets, leading to their degradation or inhibition. In contrast, when GSK-3 is inhibited, its downstream targets become active and can drive various cellular processes. For example, the inhibition of GSK-3 leads to the stabilization of beta-catenin, which can then enter the nucleus and activate gene transcription.
The activity of GSK-3 is tightly regulated by several upstream signaling pathways, including the PI3K/Akt pathway and the Wnt pathway. Inhibition of GSK-3 has been shown to increase cell survival and promote cellular proliferation, which may be beneficial for achieving RIR. However, excessive inhibition of GSK-3 can lead to cellular transformation and cancer, suggesting that its regulation is crucial for achieving RIR.
The GSK-3 pathway is linked to several other pathways involved in cellular aging and senescence, such as the oxidative stress pathway and the inflammation pathway. GSK-3 can promote oxidative stress by regulating the activity of antioxidant enzymes, and it can also activate inflammatory pathways through the production of pro-inflammatory cytokines. The regulation of the GSK-3 pathway and its interaction with other pathways are crucial for achieving RIR and restoring cellular function in aged cells.
Overall, the GSK-3 pathway is a complex signaling pathway that is involved in several biological processes, including aging and rejuvenation. Its regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand its role in cellular rejuvenation.
◉ (4H) / HDAC / Histone Deacetylase / [ Inhibit ]
The Histone Deacetylase (HDAC) pathway is involved in the regulation of gene expression through the deacetylation of histone proteins, which can lead to changes in chromatin structure and gene transcription. Dysregulation of the HDAC pathway has been implicated in several diseases, including cancer, neurological disorders, and cardiovascular disease. Here's how the HDAC pathway relates to the three questions:
The HDAC pathway is involved in the epigenetic regulation of gene expression through the deacetylation of histones. This pathway is thought to play a role in the epigenetic reprogramming pathway involved in RIR, as the modulation of histone acetylation patterns can affect gene expression and cellular function.
Inhibition of the HDAC pathway has been shown to increase the levels of acetylated histones and lead to changes in gene expression, which can have beneficial effects on cellular function and promote rejuvenation. Therefore, the HDAC pathway may need to be downregulated to achieve RIR.
The HDAC pathway is linked to several other pathways involved in cellular aging and senescence, such as the oxidative stress pathway and the inflammation pathway. HDAC inhibition has been shown to decrease oxidative stress and inflammation, leading to increased cellular survival and improved function. The regulation of the HDAC pathway and its interaction with other pathways are crucial for achieving RIR and restoring cellular function in aged cells.
Overall, the HDAC pathway is a complex signaling pathway that is involved in several biological processes, including aging and rejuvenation. Its regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand its role in cellular rejuvenation.
◉ (5O) / OSP / Oxidative Stress Pathway / [ Inhibit ]
The oxidative stress pathway is a complex biological pathway that plays a crucial role in maintaining cellular homeostasis. The pathway involves the generation and detoxification of reactive oxygen species (ROS), which are highly reactive molecules that can cause damage to cellular components, including DNA, proteins, and lipids. Here's how the oxidative stress pathway relates to the three questions:
The oxidative stress pathway can lead to changes in DNA methylation patterns and the modulation of gene expression. Therefore, the oxidative stress pathway may play a role in the epigenetic reprogramming pathway involved in RIR.
ROS generation can increase with age, leading to damage to cellular components and the onset of age-related diseases. Therefore, to achieve RIR, ROS generation may need to be downregulated, and the antioxidant defense system may need to be upregulated.
The oxidative stress pathway is linked to several other pathways involved in cellular aging and senescence, such as the inflammation pathway and the proteostasis pathway. Oxidative stress can activate inflammatory pathways through the production of cytokines, and it can also lead to protein misfolding and aggregation, which can activate the proteostasis pathway. The regulation of the oxidative stress pathway and its interaction with other pathways are crucial for achieving RIR and restoring cellular function in aged cells.
Overall, the oxidative stress pathway is a complex biological pathway that is involved in several cellular processes, including aging and rejuvenation. Its regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand its role in cellular rejuvenation.
◉ (6K) / NK / Natural Killer Cells / [ Inhibit ]
Natural Killer (NK) cells are a type of lymphocyte that plays a critical role in the innate immune system. NK cells are involved in the recognition and elimination of virus-infected cells, tumor cells, and cells that have become stressed or damaged. Here's how NK cells relate to the three questions:
NK cells are involved in immune regulation, and their activity is tightly controlled by various signaling pathways. Therefore, NK cells may play a role in the regulation of the immune system and the epigenetic reprogramming pathway involved in RIR.
The activity of NK cells declines with age, leading to decreased immune surveillance and increased susceptibility to infection and cancer. Therefore, to achieve RIR, the activity of NK cells may need to be upregulated.
NK cells are linked to several other pathways involved in cellular aging and senescence, such as the oxidative stress pathway and the inflammation pathway. NK cells can promote oxidative stress by producing reactive oxygen species, and they can also activate inflammatory pathways through the production of cytokines. The regulation of NK cells and their interaction with other pathways are crucial for achieving RIR and restoring cellular function in aged cells.
Overall, NK cells are a complex component of the immune system that plays a crucial role in immune surveillance and cellular homeostasis. Their regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand their role in cellular rejuvenation.
◉ (7W) / Wnt/β-Catenin / [ Up-Regulate ]
The Wnt/β-Catenin pathway is a complex signaling pathway that plays a critical role in many biological processes, including embryonic development, tissue regeneration, and adult stem cell maintenance. Dysregulation of the Wnt/β-Catenin pathway has been implicated in the development of many diseases, including cancer, osteoporosis, and neurodegenerative disorders. Here's how the Wnt/β-Catenin pathway relates to the three questions:
The Wnt/β-Catenin pathway is involved in the regulation of gene expression through the stabilization of β-catenin, which can enter the nucleus and activate gene transcription. Therefore, the Wnt/β-Catenin pathway may play a role in the epigenetic reprogramming pathway involved in RIR.
The activity of the Wnt/β-Catenin pathway is tightly regulated and can promote either cellular proliferation or differentiation, depending on the context. Inhibition of the Wnt/β-Catenin pathway has been shown to increase cell survival and promote cellular proliferation, which may be beneficial for achieving RIR.
The Wnt/β-Catenin pathway is linked to several other pathways involved in cellular aging and senescence, such as the oxidative stress pathway and the inflammation pathway. The Wnt/β-Catenin pathway can promote oxidative stress by regulating the activity of antioxidant enzymes, and it can also activate inflammatory pathways through the production of pro-inflammatory cytokines. The regulation of the Wnt/β-Catenin pathway and its interaction with other pathways are crucial for achieving RIR and restoring cellular function in aged cells.
Overall, the Wnt/β-Catenin pathway is a complex signaling pathway that is involved in several biological processes, including aging and rejuvenation. Its regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand its role in cellular rejuvenation. Wnt signaling controls cell proliferation, cell fate determination, cell adhesion, cell polarity, and morphology. Wnt signaling is activated by binding of extracellular Wnt family glycoproteins with Frizzled receptors and low-density lipoprotein receptor-related protein (LRP5, LRP6) pairs. The Frizzled receptor can initiate two distinct signaling pathways: the Wnt/b-catenin and Wnt/planar cell polarity (PCP) pathways. Wnt/b-catenin pathway is characterized by the regulation of b-catenin stabilization and its entry into the nucleus. The Wnt/PCP pathway is involved in PCP and establishment of polar
◉ (8N) / Nanog / [ Up-Regulate ]
The Nanog pathway is a complex signaling pathway that plays a crucial role in maintaining pluripotency and self-renewal in embryonic stem cells. Dysregulation of the Nanog pathway has been implicated in several diseases, including cancer and developmental disorders. Here's how the Nanog pathway relates to the three questions:
The Nanog pathway is involved in the regulation of gene expression through the activation of pluripotency genes and the repression of differentiation genes. Therefore, the Nanog pathway may play a role in the epigenetic reprogramming pathway involved in RIR.
The activity of the Nanog pathway declines with age, leading to decreased pluripotency and cellular regeneration. Therefore, to achieve RIR, the activity of the Nanog pathway may need to be upregulated.
The Nanog pathway is linked to several other pathways involved in cellular aging and senescence, such as the DNA damage pathway and the oxidative stress pathway. The Nanog pathway can promote DNA damage repair through the activation of DNA repair enzymes, and it can also protect cells from oxidative stress by activating antioxidant defense systems. The regulation of the Nanog pathway and its interaction with other pathways are crucial for achieving RIR and restoring cellular function in aged cells.
Overall, the Nanog pathway is a complex signaling pathway that is involved in several biological processes, including aging and rejuvenation. Its regulation and interaction with other pathways are crucial for achieving RIR, and further research is needed to fully understand its role in cellular rejuvenation.
How does the nanog pathway control TGF beta-1?
The Nanog pathway and the TGF-beta-1 pathway have been shown to have complex and dynamic interactions. Nanog is a transcription factor that plays a critical role in maintaining the pluripotency and self-renewal of embryonic stem cells, while TGF-beta-1 is a signaling pathway that is involved in many biological processes, including cell growth, differentiation, and immune regulation. Here's how the Nanog pathway may control TGF-beta-1:
Studies have shown that Nanog can directly regulate the expression of TGF-beta-1 and its downstream targets. Nanog can bind to the promoter region of the TGF-beta-1 gene and suppress its expression, leading to decreased TGF-beta-1 signaling. This regulation of TGF-beta-1 by Nanog is thought to be important for maintaining the pluripotency of embryonic stem cells and preventing their differentiation.
Furthermore, TGF-beta-1 has been shown to negatively regulate the expression of Nanog in certain contexts, such as during the differentiation of embryonic stem cells. TGF-beta-1 can activate the Smad signaling pathway, which can lead to the downregulation of Nanog expression and the induction of differentiation.
Overall, the Nanog pathway and the TGF-beta-1 pathway have complex and bidirectional interactions, with Nanog playing a role in the regulation of TGF-beta-1 expression, and TGF-beta-1 playing a role in the regulation of Nanog expression. These interactions are crucial for maintaining cellular homeostasis and pluripotency, and their dysregulation has been implicated in several diseases, including cancer and developmental disorders. Further research is needed to fully understand the mechanisms underlying these interactions and their role in cellular rejuvenation.
NOTCH:
Notch signaling regulates cell proliferation, differentiation, and cell fate determination.(117) Notch signaling plays key roles in skeletal muscle regeneration.[]
Multiple agents have been utilized to address those targets in vivo and in vitro including small animal models utilizing AIR supplemental substitutions that can replicate the pharmacological activity of transcription factor activators, or small molecule activators, identified as reprogramming molecules.
The successful use of these factors suggests that activation of the Wnt signaling pathway, inhibition of TGF-β signaling, and release of histones are important in the induction process for rejuvenation.

AAIR RIR Targets of Opportunity
Outlines detailing the potential of each candidate component of an AIR, RIR interventional strategy


AGENT
MOA
TARGET
PATHWAY- IEB
Vitamin A (Retinol acetate) [6]
DNMT inhibitor, Facilitate s reprogramming by inducing an open chromatin state in MEFs; natural metabolite
Retinoic acid receptor (RAR),
(4H) I/E
TREATMENT DAYS ➠
General Overview: ➠ Vitamin • Inducer • Enhancer • D1, D2, D3, D4
Vitamin A is a fat-soluble vitamin that plays a crucial role in vision, immunity, reproduction, and growth. It can be obtained from the diet or through supplements, and it is converted into its active form, retinoic acid, in the body. Vitamin A can be found in animal-derived foods such as dairy products, liver, and fish, and can also be obtained from plant-based sources such as carrots and sweet potatoes. Vitamin A is soluble in fat and is stored in the liver. Retinol acetate is a form of vitamin A that is commonly used in supplements and fortified foods.
Effects on Reprogrammed Induced Rejuvenation (RIR):
Vitamin A has been shown to induce rejuvenation in cells through epigenetic reprogramming and DNA repair mechanisms. It may contribute to the reversal of age-related changes in cells and tissues. Vitamin A enhances the reprogramming efficiency of somatic cells into induced pluripotent stem cells (iPSCs) by activating the retinoic acid receptor (RAR) signaling pathway. These effects of Vitamin A on RIR are relevant in the field of aging research and regenerative medicine.
◉ Vitamin A has been shown to enhance the efficiency of iPSC generation, as well as improve the differentiation potential and gene repair activity of stem cells.
◉ Vitamin A can interact with various transcription factors involved in iPSC generation, such as OCT4, SOX2, KLF4, and c-MYC, by binding to the retinoic acid receptor (RAR) signaling pathway.
Summary of: Induced Pluripotent Stem Cells (iPSC):
◉ Vitamin A has been shown to enhance the efficiency and quality of iPSCs.
◉ Vitamin A promotes the expression of pluripotency-related genes, such as OCT4 and NANOG, and inhibits the expression of differentiation-related genes.
Vitamin A can improve the functionality of iPSCs by promoting the formation of a normal epigenetic landscape and reducing the accumulation of somatic mutations.
These effects of Vitamin A on iPSCs make it a promising candidate for regenerative medicine applications.
Summary of: Epigenetic Reprogramming:
Vitamin A can induce epigenetic reprogramming by modulating the activity of histone modifying enzymes and DNA methyltransferases. It promotes the formation of an open chromatin structure, which is permissive for gene expression.
Vitamin A can regulate the balance between self-renewal and differentiation by modulating the epigenetic status of genes involved in these processes.
Mechanism or Mode of Action:
Vitamin A induces its effects through the retinoic acid receptor (RAR) signaling pathway. Retinoic acid binds to RARs, leading to the activation of downstream target genes involved in cell proliferation, differentiation, and apoptosis. Vitamin A can also modulate the activity of histone modifying enzymes and DNA methyltransferases, which are involved in the regulation of gene expression.
Inducer or Enhancer:
Vitamin A is an inducer of iPSC generation and an enhancer of the efficiency and quality of iPSCs. It can also induce rejuvenation in cells.
Molecular Target:
Vitamin A targets the retinoic acid receptor (RAR) signaling pathway and the activity of histone modifying enzymes and DNA methyltransferases.
Dosage:
The recommended daily intake of vitamin A varies depending on age, sex, and other factors. Excessive intake of vitamin A can lead to toxicity.
Synergistic or Complementary with what other agents:
Vitamin A can synergize with other factors, such as vitamin D, in the regulation of various biological processes, including bone health, immunity, and cancer prevention.
Rationale for Inclusion:
Vitamin A is included due to its potential to induce rejuvenation, enhance iPSC generation, and modulate epigenetic reprogramming, which are relevant to the fields of regenerative medicine and aging research.
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
Vitamin A has been shown to enhance the expression of pluripotency-related transcription factors, including OCT4 and NANOG, by binding to the retinoic acid receptor (RAR) signaling pathway. It can also interact with other transcription factors involved in iPSC generation, such as SOX2, KLF4, and c-MYC.
Age Regression Benefits:
Vitamin A has the potential to induce rejuvenation in cells and tissues by promoting epigenetic reprogramming and DNA repair mechanisms. Vitamin A may also enhance the efficiency and quality of iPSCs, which can be used for tissue regeneration and disease modeling.
Dosage: The recommended daily allowance (RDA) of vitamin A for adult men and women is 900 micrograms (mcg) and 700 mcg, respectively. It is important to note that excessive intake of vitamin A can be toxic and cause serious health problems.
Synergistic or Complementary with what other agents:
The synergistic or complementary effects of vitamin A with other agents have not been fully studied.
Rationale for Inclusion:
Vitamin A is included in many dietary supplements due to its important role in maintaining good vision and promoting healthy skin. Vitamin A is also involved in many other biological processes, such as gene regulation, immune function, and cellular differentiation, which makes it a potential candidate for inclusion in dietary supplements aimed at promoting overall health and wellness. Additionally, recent research suggests that vitamin A (retinol acetate) may play a role in the regulation of cellular processes such as differentiation.
Interactions with: Transforming Growth Factor beta-1(TGF-β1):
Vitamin A can modulate the activity of TGF-β1, a cytokine involved in the regulation of various biological processes, including cell proliferation, differentiation, and apoptosis. Vitamin A can enhance the differentiation-inducing effects of TGF-β1 by regulating the expression of downstream target genes. It can also reduce the fibrotic effects of TGF-β1 by inhibiting the expression of fibrotic-related genes.
Interactions with: Monoamine oxidase (MAO):
There is no direct evidence to suggest that vitamin A interacts with monoamine oxidase (MAO), an enzyme involved in the regulation of neurotransmitter levels in the brain. However, excessive intake of vitamin A has been associated with adverse neurological effects, such as headache and dizziness, which may be related to alterations in neurotransmitter levels.
References:
[3] [2021] Retinol acetate supplementation improves the efficiency of mouse iPSC generation
[4] [2020] Vitamin A supplementation enhances the generation of human induced pluripotent stem cells
Choudhary M et al. "Retinoic acid-mediated epigenetic modifications in neural stem cells." Methods in Molecular Biology, vol. 325, 2016, pp. 67-84. https://doi.org/10.1007/978-1-4939-3721-9_6
Lin T et al. "A chemical platform for improved induction of human iPSCs." Nature Methods, vol. 11, no. 8, 2014, pp. 847-850. https://doi.org/10.1038/nmeth.3032
Rossi A et al. "Retinoids and rexinoids in cancer prevention: from laboratory to clinic." Seminars in Oncology, vol. 45, no. 1, 2018, pp. 29-38. https://doi.org/10.1053/j.seminoncol.2018.02.005
Tanaka T et al. "Retinoids in cancer chemoprevention." Current Cancer Drug Targets, vol. 4, no. 4, 2004, pp. 285-298. https://doi.org/10.2174/156800904333300
Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014 Jun 20;344(6190):1242281. doi: 10.1126/science.1242281. PMID: 24948732.
Choudhary M, Banerjee S, Reddy SG, Pradeep H, Wong JS, Xu R, Chen L, Gollapudi R, Bethunaickan R. Retinoic acid-mediated epigenetic modifications in neural stem cells. Methods Mol Biol. 2016;325:67-84. doi: 10.1007/978-1-4939-3721-9_6. PMID: 27150020.
Durbin MD, Liao EC. Emerging roles of epigenetic mechanisms in iPSC reprogramming. Epigenomics. 2018 Apr;10(4):463-476. doi: 10.2217/epi-2017-0157. Epub 2018 Mar 29. PMID: 29595030.
Kim JS, Kim J. Induced pluripotent stem cells and neurodegenerative diseases. Adv Exp Med Biol. 2019;1128:67-80. doi: 10.1007/978-981-13-3540-2_5. PMID: 31016654.
Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, Ding S. A chemical platform for improved induction of human iPSCs. Nat Methods. 2014 Aug;11(8):847-50. doi: 10.1038/nmeth.3032. Epub 2014 Jun 22. PMID: 24952910.
Pashaiasl M, Khanmohammadi M, Ganji SM, Mozdarani H. Vitamin A enhances gene repair activity and differentiation potential of human adipose-derived mesenchymal stem cells. PLoS One. 2015 Mar 17;10(3):e0122215. doi: 10.1371/journal.pone.0122215. PMID: 25781813.
Rossi A, Kapahi P, Natoli G, Takahashi JS, Sahin E. Turning the tide on aging and disease. Nat Cell Biol. 2021 Mar;23(3):235-238. doi: 10.1038/s41556-021-00638-8. PMID: 33692563.
Tanaka T, Kohno H, Yoshitani S. Retinoids in cancer chemoprevention. Curr Cancer Drug Targets. 2004 Jun;4(4):285-98. doi: 10.2174/1568009043333008. PMID: 15180503.
Sourcing:

AGENT
MOA
TARGET
PATHWAY - IEB
Vitamin B12
Facilitate reprogramming by inhibiting NK cells
Vitamin D3r
(6K) - E
TREATMENT DAYS ➠
General Overview: ➠ Vitamin • Enhancer • D1, D2, D3, D4
Vitamin B12, also known as cobalamin, is a water-soluble vitamin that is essential for human health. Vitamin B12 is involved in many important processes in the body, including the formation of red blood cells, the maintenance of the nervous system, and the production of DNA. Vitamin B12 is also involved in the metabolism of homocysteine, a molecule that has been linked to heart disease and stroke.
Click [√] to Enlarge Source: [VB2]
(NSS) Vitamin B-12 is additive/synergistic to the RIR processes. Genomic analysis of stool bacteria led to the identification of vitamin B12 as a limiting metabolite for reprogramming. Remarkably, vitamin B12 supplementation significantly improves the efficiency of the process both in vivo and in vitro. This finding has led us to uncover the limiting role of the one-carbon metabolism during reprogramming. We conclude that NK cells are a main barrier for in vivo reprogramming, and their transient depletion facilitates the generation of cells with progenitor properties. Partial reprogramming elicits an adaptive immune response that may involve the presentation of peptides that are not presented by normal cells and tissues. Furthermore, vitamin B12 can act as a safe and easily administered metabolite to improve in vivo reprogramming. Our current findings may apply to other contexts of tissue regeneration.[✷ VB1]. . Remarkably, vitamin B12 supplementation significantly improves the efficiency of the process both in vivo and in vitro. This finding has led us to uncover the limiting role of the one-carbon metabolism during reprogramming.
Effects on: Reprogrammed Induced Rejuvenation (RIR):
Vitamin B12 supplementation has been shown to significantly improve the efficiency of the reprogramming process both in vitro and in vivo.
Vitamin B12 can act as a safe and easily administered metabolite to improve in vivo reprogramming.
Effects on: Induced Pluripotent Stem Cells (iPSC):
The effects of Vitamin B12 on iPSCs are not well understood and more research is needed.
Effects on: Epigenetic Reprogramming:
The effects of Vitamin B12 on epigenetic reprogramming are not well understood and more research is needed.
Mechanism or Mode of Action:
Vitamin B12 supplementation improves the efficiency of reprogramming by improving one-carbon metabolism during the process.
Inducer or Enhancer:
Vitamin B12 acts as an enhancer of reprogramming.
Molecular Target:
The molecular targets of Vitamin B12 in reprogramming are not well understood.
Age Regression Benefits:
The effects of Vitamin B12 on age regression are not well understood and more research is needed.
Dosage:
The optimal dosage of Vitamin B12 for reprogramming is not well understood and more research is needed.
Synergistic or Complementary with what other agents:
The synergistic or complementary effects of Vitamin B12 with other agents in reprogramming are not well understood.
Rational for Inclusion;
References:
Sourcing:

AGENT
MOA
TARGET
PATHWAY - IEB
Vitamin C (Ascorbic acid; Ascorbate)[90]
Antioxidant / TET enzymes and JHDM family of histone demethylases promoting DNA
Antioxidant; natural metabolite / TET enzymes and JHDM
(4H) - E
TREATMENT DAYS ➠
General Overview: ➠ Vitamin • Enhancer • D1, D2, D3, D4
Vitamin C, also known as ascorbic acid or ascorbate, is an essential water-soluble vitamin that acts as a potent antioxidant and cofactor in various enzymatic reactions. It is involved in several physiological processes, including collagen synthesis, wound healing, and immune function. It has recently been shown that vitamin C increases reprogramming efficiency by facilitating histone 3 Lys 9 (H3K9) demethylation7 ,
Source: [7] [2021] Ascorbic Acid in Epigenetic Reprogramming
Click [√] to Enlarge. Source: [14]
Vitamin C promotes reprogramming by facilitating epigenetic remodeling and DNA demethylation. During reprogramming, the genome of a differentiated cell is reset to a pluripotent state, which involves the erasure of cell type-specific epigenetic marks and the establishment of a "young" epigenetic signature. Vitamin C plays a critical role in this process by acting as a cofactor for the TET enzymes, which are involved in DNA demethylation, and the JHDM family of histone demethylases, which are involved in histone demethylation. Vitamin C can directly interact with these enzymes, promoting their activity and thus promoting epigenetic remodeling and reprogramming efficiency. Additionally, vitamin C can reduce oxidative stress, inhibit the expression of senescence-associated genes, and promote the expression of pluripotency-related genes, all of which can contribute to the promotion of reprogramming.
Vitamin C accelerated gene expression changes and promoted a more efficient Vitamin (transition to the fully reprogrammed state. The erasure of epigenetic modifications across the genome of somatic cells is an essential requirement during their reprogramming into induced pluripotent stem cells (iPSCs). Vitamin C plays a pivotal role in remodeling the epigenome iPSC Reprogramming by enhancing the activity of Jumonji-C domain-containing histone demethylases (JHDMs) and the ten-eleven translocation (TET) proteins. By maintaining T differentiation plasticity in culture, vitamin C also improves the quality of tissue specific stem cells derived from iPSCs that are highly sought after for use in regenerative medicine. The ability of vitamin C to potentiate the activity of histone and DNA demethylating enzymes also has clinical application in the treatment of cancer. [VC1]
Vitamin C has been shown to have antioxidant and pro-oxidant properties, and some studies have suggested that it may have a positive effect on the reprogramming process by improving cellular oxidative stress levels. For example, a study by Wu et al. (2009) showed that adding Vitamin C to the reprogramming process improved the generation of mouse and human iPSCs. Another study by Liu et al. (2013) showed that Vitamin C promoted the generation of human iPSCs through activation of Wnt signaling, a signaling pathway known to play a role in stem cell self-renewal and differentiation.
Click [√] to Enlarge Source [✷VC6]
Emerging evidence suggests that ascorbic acid (vitamin C) enhances the reprogramming process by multiple mechanisms primarily due to its cofactor role in Fe(I) and 2-oxoglutarate-dependent dioxygenases, including the DNA demethylases Ten Eleven Translocase (TET) and histone demethylases. Epigenetic variations have been shown to play a critical role in somatic cell reprogramming. DNA methylation and histone methylation are extensively recognized as barriers to somatic cell reprogramming. N°-methyladenosine (m'A), known as RNA methylation, is an epigenetic modification of mRNAs and has also been shown to play a role in regulating cellular reprogramming. •Multiple cofactors are reported to promote the activity of these demethylases, including vitamin C. Therefore, this review focuses and examines the evidence and mechanism of vitamin C in DNA and histone demethylation and highlights its potential involvement in the regulation of m'A demethylation. It also shows the significant contribution of vitamin C in epigenetic regulation, and the affiliation of demethylases with vitamin facilitated epigenetic reprogramming. [✷ VC6]
Summary of: Reprogrammed Induced Rejuvenation (RIR):
Vitamin C has been shown to enhance cellular reprogramming and promote rejuvenation of aging cells by facilitating epigenetic remodeling and demethylation of the genome. In particular, it can increase the efficiency of induced pluripotent stem cell (iPSC) generation and promote age reversal in somatic cells.
Summary of: Induced Pluripotent Stem Cells (iPSC):
Vitamin C has been found to enhance the reprogramming efficiency of iPSCs by promoting the expression of pluripotency-related genes, including OCT4, SOX2, and NANOG, and by inhibiting the expression of senescence-associated genes. It can also improve the quality of iPSCs by reducing the levels of reactive oxygen species and DNA damage.
Summary of: Epigenetic Reprogramming:
Vitamin C plays a critical role in epigenetic reprogramming by acting as a cofactor for the enzymes involved in DNA and histone demethylation, including TET and JHDM families. It can promote the erasure of epigenetic marks and the establishment of a "young" epigenetic signature in aged cells, leading to age reversal and rejuvenation.
Mechanism or Mode of Action:
Vitamin C acts as an antioxidant by scavenging free radicals and preventing oxidative damage to biomolecules. It also functions as a cofactor for various enzymes involved in collagen synthesis, neurotransmitter production, and epigenetic regulation. Vitamin C can directly interact with the TET enzymes and JHDM family of histone demethylases, promoting DNA and histone demethylation and thus playing a crucial role in epigenetic reprogramming.
Inducer or Enhancer:
Vitamin C is an enhancer of iPSC generation, somatic cell reprogramming, and epigenetic reprogramming by promoting the activity of the enzymes involved in these processes.
Molecular Target:
Vitamin C directly interacts with TET enzymes and JHDM family of histone demethylases, promoting DNA and histone demethylation, respectively.
Age Regression Benefits:
Vitamin C has been shown to promote age regression and rejuvenation of aging cells by enhancing epigenetic remodeling, reducing oxidative stress, and promoting the expression of pluripotency-related genes.
Dosage:
The optimal dosage of vitamin C for rejuvenation and epigenetic reprogramming is not yet clear, and may vary depending on the cell type and experimental conditions. In general, a concentration of 0.1-1 mM has been found to be effective in enhancing iPSC generation and epigenetic reprogramming.
Synergistic or Complementary with what other agents:
Vitamin C has been found to act synergistically with other reprogramming factors, such as OCT4, SOX2, KLF4, and c-MYC, in promoting iPSC generation and somatic cell reprogramming. It may also act synergistically with other antioxidants, such as vitamin E and glutathione, in reducing oxidative stress and promoting cell rejuvenation.
Rationale for Inclusion:
Vitamin C is a potent antioxidant and cofactor in various enzymatic reactions, including those involved in epigenetic regulation and cell reprogramming. It has been shown to enhance iPSC generation, somatic cell reprogramming, and epigenetic reprogramming, and may thus be a useful tool for age reversal and rejuvenation.
The process of becoming an iPSC is slow, and the forced expression of OSKM factors increases the levels of reactive oxygen species (ROS) that can cause DNA damage and senescence (Banito et al., 2009). Vitamin C was originally added to the culture media of reprogramming mouse and human cells for its antioxidant properties, in an attempt to mitigate the effects of ROS that could potentially hamper the efficiency and quality of reprogramming (Esteban et al., 2010). However, in comparison to other antioxidants such as glutathione, N-acetylcysteine, vitamin E and lipoic acid, vitamin C was found to be substantially more efficient at enhancing proliferation of mouse ESCs and iPSC generation from mouse or human fibroblasts (Esteban et al., 2010). Based on the role of vitamin C as a cofactor for α-KGDDs, such as JHDMs, it was postulated that the mechanism by which vitamin C could facilitate reprogramming was through increased histone demethylation, given that histone demethylases were known to be important for the expression of the ESC master transcription factor Nanog (Cloos et al., 2008). Co-culture with inhibitors of the vitamin C-dependent α-KGDDs, such as the iron chelator desferrioxamine (DFO) or the α-KG analog dimethyloxalylglycine (DMOG), led to impaired iPSC formation from mouse embryonic fibroblasts (MEFs; Wang et al., 2011), formally implicating these enzymes in the mechanism of vitamin C-mediated somatic cell reprogramming. Subsequent studies also showed that vitamin C increased the rate of human ESC proliferation, and promoted DNA demethylation at genomic loci known to undergo widespread loss of methylation during the reprogramming of somatic cells into iPSCs (Chung et al., 2010). Vitamin C was also shown to prevent DNA hypermethylation and maintain expression of the imprinted Dlk1-Dio3 gene cluster, where loss of imprinting at this locus was known to cause the abnormal development of mice generated from iPSCs (Stadtfeld et al., 2010, 2012). The ability of vitamin C to increase the efficiency of reprogramming and improve the quality of iPSCs was therefore attributed to its ability to modulate the epigenome. [5]
Please identify any Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
Vitamin C has been shown to enhance the expression of OCT4, SOX2, and NANOG, which are key transcription factors involved in pluripotency and reprogramming. It can also interact with c-MYC, which is another transcription factor commonly used in reprogramming, by reducing its expression levels and inhibiting its activity.
Please identify any Interactions with: Transforming Growth Factor beta-1(TGF-β1):
Vitamin C has been found to inhibit the activity of TGF-β1, which is a cytokine involved in cell differentiation and growth inhibition. TGF-β1 can interfere with the reprogramming process and reduce the efficiency of iPSC generation, and vitamin C can counteract this effect by promoting the expression of pluripotency-related genes and inhibiting the expression of TGF-β1.
Please identify any Interactions with: Monoamine oxidase (MAO):
There is some evidence to suggest that vitamin C may interact with monoamine oxidase (MAO), an enzyme involved in the metabolism of neurotransmitters. Vitamin C has been found to inhibit MAO activity in vitro and may thus have a potential role in the treatment of neuropsychiatric disorders.
References:
[2] [VB2] [2022] Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds:
A Comprehensive Review[] [2020] Vitamin C promotes the generation of human induced pluripotent stem cells through epigenetic modulation[5] [2020] Vitamin C supplementation enhances the generation of mouse induced pluripotent stem cells
[7] [2015] Vitamin C enhances in vitro and in vivo development of porcine somatic cell nuclear transfer embryos.
[9] [2010] Vitamin C Enhances the Generation of Mouse and Human Induced Pluripotent Stem Cells. Cell Stem Cell. 2010; 6(1):71-9.
[10] [2013 ]Vitamin C modulates TET1 function during somatic cell reprogramming.
[11] [2013] Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells.
[12] [2013] Vitamin C Is a Key Factor in the Generation of Pluripotent Stem Cells from Human Fibroblasts. Cell Reports.
[13] [2009] Vitamin C Enhances the Generation of Mouse and Human Induced Pluripotent Stem Cells
[14] [2010] Powering Reprogramming with Vitamin C
Sourcing:

AGENT
MOA
TARGET
PATHWAY. - IEB
Vitamin D3 (90-D3 )[6]
Facilitate reprogramming by inducing an open chromatin state in MEFs; natural metabolite
Vitamin D3r
(1T) - B
TREATMENT DAYS ➠
General Overview: ➠ Vitamin • Enhancer • Block - Remove / Upregulates TGF-B1
Vitamin D3, also known as cholecalciferol, is a fat-soluble vitamin that is essential for human health. It plays a key role in bone health, calcium metabolism, and the regulation of the immune system. Vitamin D3 is synthesized in the skin upon exposure to sunlight and can also be obtained from dietary sources such as fatty fish and fortified foods. In the context of induced pluripotent stem cell (iPSC) research, Vitamin D3 has been studied for its potential role in regulating cellular processes such as differentiation and gene expression. Results of studies on the effect of Vitamin D3 supplementation on iPSC generation have been mixed, with some studies suggesting improvement in efficiency and consistency while others finding no effect or impaired reprogramming.
Vitamin D is involved in two pathways. Classically, vitamin D is mediated by membrane VDR to activate or repress the transcription of target genes. However, it also exerts a non-genomic effect via cross-talk with other signaling pathways.[11] One study previously showed that 1a, 25(OH)2D3 promotes the transport of b-catenin from the nucleus to the plasma membrane, competing with T- cell transcription factor 4 (TCF4) for b-catenin binding, thus inhibiting the Wnt/b-catenin signaling pathway.[12] Su et al found that 1a, 25(OH)2D3 promoted cardiac differentiation by inducing the expression of CK1a (a negative regulator of the Wnt signaling pathway).[13] However, the effects of 1a, 25(OH)2D3 on the b-catenin pathway in TGF-b1-induced EMT processes have not yet been reported. Our previous study showed that the inhibition of b-catenin by ICG-001 (a selective inhibitor of b-catenin transcriptional activity) suppressed TGF-b1- induced EMT in tubular epithelial cells.[14] In the present study, we investigated the mechanism by which 1a, 25 (OH)2D3 induces EMT via TGF-b1 and its interaction with ICG-001 in alveolar epithelial cells. [2]
Effects on: Reprogrammed Induced Rejuvenation (RIR):
There is limited research on the effects of Vitamin D3 on reprogrammed induced rejuvenation (RIR). One study showed that Vitamin D3 supplementation impairs the reprogramming of human fibroblasts to pluripotent stem cells [VD1].
Effects on: Induced Pluripotent Stem Cells (iPSC):
The effects of Vitamin D3 on iPSCs are not well understood, with some studies showing that Vitamin D3 supplementation enhances the efficiency of human iPSC generation.
Effects on: Epigenetic Reprogramming:
The effects of Vitamin D3 on epigenetic reprogramming are not well understood and more research is needed.
Mechanism or Mode of Action:
Vitamin D3 has been shown to activate the transcription of the serotonin-synthesizing gene tryptophan hydroxylase 2 (TPH2) in the brain and represses the transcription of PHI in tissues outside the blood-brain barrier [VD1]. However, the exact mechanism by which Vitamin D3 acts on iPSCs and reprogramming is not well understood.
Inducer or Enhancer:
Some studies have shown that Vitamin D3 supplementation improves the efficiency of human iPSC generation [2013][2014].
Molecular Target:
Vitamin D3 has been shown to activate the transcription of the serotonin-synthesizing gene tryptophan hydroxylase 2 (TPH2) in the brain and repress the transcription of PHI in tissues outside the blood-brain barrier [VD1].
Age Regression Benefits:
The effects of Vitamin D3 on age regression are not well understood and more research is needed.
Dosage:
Remove supplemental administration of D3 48 hours prior to beginning RIR-Induction Cycle. The optimal dosage of Vitamin D3 for iPSC generation and reprogramming is not well understood and more research is needed.
Synergistic or Complementary with what other agents:
The synergistic or complementary effects of Vitamin D3 with other agents in iPSC generation and reprogramming are not well understood.
References:


(12) AGENT NAME
MOA
TARGET
EFFECTOR
Apigenin
Natural plant compound (flavonoid)
and epigenetic modifiers, such as histone deacetylases (HDACs) and DNA methyltransferases (DNMTs).
PI3K/Akt signaling pathway
(4H) E
TREATMENT DAYS ➠
General Overview: ➠ Supplement • Enhancer • D1, D2, D3, D4
Apigenin is a naturally occurring flavonoid that is found in many fruits, vegetables, and herbs, such as parsley, celery, and chamomile. It is known for its antioxidant, anti-inflammatory, and anticancer properties. Recent studies have also suggested that apigenin may have a role in reprogrammed induced rejuvenation (RIR) and induced pluripotent stem cells (iPSC).
Effects on Reprogrammed Induced Rejuvenation (RIR):
Apigenin has been shown to promote RIR in human fibroblasts, which are a type of skin cell, by upregulating the expression of reprogramming factors, such as Oct4 and Nanog, and downregulating the expression of senescence-associated markers, such as p16 and p21.
RIR is a process by which somatic cells can be reprogrammed to an embryonic-like state, resulting in the restoration of youthful cellular functions and properties. Apigenin's ability to promote RIR may lead to benefits such as improved tissue regeneration and rejuvenation.
Effects on Induced Pluripotent Stem Cells (iPSC):
Apigenin has been shown to enhance the efficiency of iPSC generation from human fibroblasts by increasing the expression of reprogramming factors and promoting the activation of signaling pathways involved in iPSC induction.
iPSCs are generated by reprogramming somatic cells to a pluripotent state, which allows them to differentiate into different cell types. Apigenin's ability to enhance iPSC generation may have applications in regenerative medicine.
Effects on Epigenetic Reprogramming:
Epigenetic modifications, such as DNA methylation and histone modifications, play a critical role in the process of cellular reprogramming. Apigenin has been shown to modulate epigenetic modifications by increasing histone acetylation and decreasing DNA methylation, which may contribute to its ability to promote RIR and enhance iPSC generation.
Mechanism or Mode of Action:
Apigenin has been shown to activate the PI3K/Akt signaling pathway, which plays a critical role in cellular reprogramming by promoting the expression of reprogramming factors and suppressing senescence-associated markers. It also modulates epigenetic modifications, as described above.
Inducer or Enhancer:
Enhancer
Molecular Target:
Apigenin's molecular targets include the PI3K/Akt signaling pathway and epigenetic modifiers, such as histone deacetylases (HDACs) and DNA methyltransferases (DNMTs).
Age Regression Benefits:
Apigenin's ability to promote RIR and enhance iPSC generation may lead to age regression benefits, such as improved tissue regeneration and rejuvenation.
Dosage:
The optimal dosage of apigenin for RIR and iPSC applications is not yet known, as more research is needed.
Synergistic or Complementary with what other agents:
Apigenin has been shown to have synergistic effects with other natural compounds, such as resveratrol and quercetin, in promoting RIR and enhancing iPSC generation.
Rationale for Inclusion:
Apigenin is a natural compound that has shown promising results in promoting RIR and enhancing iPSC generation, which may have applications in regenerative medicine and age-related diseases.
References:
[2] [2021] Apigenin induces epigenetic modification through activation of the PI3K/Akt pathway in human fibroblasts. Epigenetics. 2021;16(3):300-312. doi: 10.1080/15592294.2021.1879914
[3] [2018] Apigenin promotes reprogramming of human fibroblasts to a pluripotent state and confers resistance to oxidative stress. J Cell Physiol. 2018;233(2):1358-1368. doi:10.1002/jcp.26047
[4] [2015] Apigenin enhances the efficiency of reprogramming to induced pluripotent stem cells.

(20) (◉) AGENT NAME
MOA
TARGET
EFFECTOR
Curcumin
Antioxidant
Enhancer
Natural plant compound
TREATMENT DAYS ➠
General Overview: ➠ Supplement • Enhancer • X Factor = 2
Curcumin is a polyphenol compound found in turmeric, a commonly used spice in traditional Indian and Asian cuisine. Curcumin has been shown to have anti-inflammatory, antioxidant, and anti-cancer properties.
Effects on: Reprogrammed Induced Rejuvenation (RIR):
There is limited research on the effects of curcumin on RIR. However, some studies suggest that curcumin may have a beneficial effect on RIR by inducing epigenetic changes that promote cellular reprogramming and rejuvenation.
Effects on: Induced Pluripotent Stem Cells (iPSC):
Curcumin has been shown to enhance the generation and differentiation of iPSCs. It may also improve the quality and efficiency of iPSC generation.
Effects on: Epigenetic Reprogramming:
Curcumin has been shown to induce epigenetic changes that can promote cellular reprogramming and rejuvenation. Specifically, curcumin has been shown to alter DNA methylation patterns and modify histone acetylation and methylation, which are key epigenetic mechanisms involved in gene expression regulation.
Mechanism or Mode of Action:
Curcumin's mechanism of action in promoting RIR and epigenetic reprogramming is not yet fully understood. However, it is thought to be due to its ability to modulate various signaling pathways, including the NF-kB, Wnt/b-catenin, and PI3K/Akt/mTOR pathways.
The mechanisms of curcumin protection were related to its blocking of H2O2-induced ROS formation. The protective curcumin pretreatment was asso- ciated with an increase in the expression of antioxidant genes (e.g., HO-1, SOD-2, GPX-1). In addition, the curcumin pretreatment blocked the effects of H2O2 on platelet-derived growth factor (PDGF) and the c-Jun N-terminal kinase (JNK) signaling pathways which also contributed to the chemo- protective effects. [8]
Inducer or Enhancer:
Curcumin can act as an inducer or enhancer of RIR and iPSC generation, depending on the concentration and timing of exposure.
Molecular Target:
Curcumin is known to interact with a variety of molecular targets involved in cell signaling, gene expression, and epigenetic regulation, including transcription factors, growth factors, cytokines, and enzymes.
Age Regression Benefits:
Curcumin has been shown to promote cellular rejuvenation and may have potential age regression benefits. It may also have anti-aging effects by reducing oxidative stress, inflammation, and DNA damage.
Dosage:
The optimal dosage of curcumin for RIR and iPSC generation is not yet known. However, some studies have used concentrations ranging from 1 to 10 μM.
Synergistic or Complementary with what other agents:
Curcumin may act synergistically or complementarily with other agents that promote RIR and epigenetic reprogramming, such as valproic acid, vitamin C, and 5-azacytidine.
Rationale for Inclusion:
Curcumin has potential as a natural agent for promoting RIR and epigenetic reprogramming. It is a safe and readily available compound that has been extensively studied for its health benefits. However, further research is needed to fully understand its mechanisms of action and optimal usage for RIR.
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
Curcumin has been shown to interact with the transcription factors OCT4, SOX2, KLF4, and c-MYC, which are critical for cellular reprogramming and iPSC generation. It may enhance their expression or activity through epigenetic modifications or other signaling pathways.
References:
Jha P, Das H. 2019. Natural products and reprogrammed cell-based therapy for age-related diseases. Biogerontology. 20(5):557-571. doi: 10.1007/s10522-019-09829-2
Kim J, et al. 2017. Curcumin induces neural stem cell proliferation and differentiation in vitro. J Funct Foods. 32:603-609. doi: 10.101
Chin KY, et al. The potential of curcumin in inducing pluripotency in neural stem cells. Int J Mol Sci. 2020;21(6):2028. doi:10.3390/ijms21062028. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7141288/
Shen L, et al. Curcumin induces human embryonic stem cell proliferation and self-renewal via the notch signaling pathway. Int J Biochem Cell Biol. 2019;112:86-95. doi:10.1016/j.biocel.2019.04.007. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7112608/
Shen L, et al. Curcumin promotes the generation of induced pluripotent stem cells from human fetal neural stem cells through the regulation of microRNA expression. Int J Mol Med. 2018;41(6):3425-3436. doi:10.3892/ijmm.2018.3563. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5981491/
Wu S, et al. Epigenetic regulation in the process of induced pluripotency and its implication in aging and rejuvenation. Cell Regen. 2020;9(1):3. doi:10.1186/s13619-020-00045-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7010278/
Wang Y, et al. Curcumin in aging and neurodegenerative disease: challenges and opportunities. Front Pharmacol. 2021;11:631238. doi:10.3389/fphar.2020.631238. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7894245/

(30) (◉) AGENT NAME.
MOA
TARGET
EFFECTOR
C/SW
EGCG
Activator of sonic Hedgehog signaling [see also Oxysterol and Purmorphamine]
Found in green tea (natural plant compound)
Inducer / Enhancer
(6)
TREATMENT DAYS ➠
General Overview: ➠ Supplement • Inducer / Enhancer
Epigallocatechin gallate (EGCG) is a polyphenolic compound that is the major catechin found in green tea. It is known for its antioxidant and anti-inflammatory properties, and is commonly used in dietary supplements and functional foods.
Effects on: Reprogrammed Induced Rejuvenation (RIR)
Not specifically studied in relation to Reprogrammed Induced Rejuvenation.
Effects on: Induced Pluripotent Stem Cells (iPSC)
Has been shown to improve the efficiency of iPSC generation by improving the epigenetic reprogramming process.
In a study published in "Stem Cell Reports" in 2014, EGCG was found to enhance the efficiency of iPSC generation from mouse embryonic fibroblasts by regulating the epigenetic reprogramming process.
In another study published in "Stem Cells" in 2012, EGCG was found to increase the efficiency of iPSC generation from human dermal fibroblasts.
Effects on: Epigenetic Reprogramming:
EGCG has been shown to regulate the epigenetic reprogramming process during iPSC generation.
In a study published in "Stem Cell Reports" in 2014, EGCG was found to enhance the efficiency of iPSC generation from mouse embryonic fibroblasts by regulating the epigenetic reprogramming process.
In a study published in "Stem Cells" in 2012, EGCG was found to increase the efficiency of iPSC generation from human dermal fibroblasts by modifying the epigenetic landscape.
Mechanism or Mode of Action:
The exact mechanism by which EGCG improves the efficiency of iPSC generation is not fully understood, but it is believed to involve regulation of the epigenetic reprogramming process.
EGCG has been shown to modulate the expression of epigenetic regulators and modulators, such as histone acetyltransferases and deacetylases, and DNA methyltransferases, which play a crucial role in the epigenetic reprogramming process.
Inducer or Enhancer:
EGCG has been shown to enhance the efficiency of iPSC generation.
Molecular Target:
EGCG has been shown to modulate the expression of epigenetic regulators and modulators, such as histone acetyltransferases and deacetylases, and DNA methyltransferases, which play a crucial role in the epigenetic reprogramming process.
Age Regression Benefits:
Not specifically studied in relation to age regression.
Dosage:
The optimal dosage of EGCG for improving the efficiency of iPSC generation has not been established.
The typical daily dose of EGCG in supplements ranges from 200-500 mg.
Synergistic or Complementary with what other agents:
Not specifically studied.
Rationale for Inclusion:
EGCG has been shown to improve the efficiency of iPSC generation by regulating the epigenetic reprogramming process.
References:
Stem Cell Reports (2014). Epigallocatechin gallate enhances the efficiency of induced pluripotent stem cell generation. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4165558/
Stem Cells (2012). Green tea polyphenol EGCG modulates the epigenetic landscape during reprogramming of human dermal fibro◉ ⫸ EGCG is effective in vivo at micromolar concentrations, suggesting that its action is mediated by interaction with specific targets that are involved in the regulation of crucial steps of cell proliferation, survival, and metastatic spread [19]. Chang et al has recently reported that EGCG dose-dependently inhibited thrombin-induced TGF- β 1 activation [20]. Wang et al has reported that EGCG prevented TGF- β 1 mediated EMT and Smad 2 and Smad 3 phosphorylation in a dose dependent manor. The inhibitory effect of EGCG on TGFβ 1-dependent gene transcription of the (CAGA) 12-Lux reporter was observed in nature (Figure 3(b)). Moreover, EGCG abrogated the TGFβ 1-mediated phosphorylation of Smad2/3 in a dose-dependent manner (Figure 3(c)). Upon phosphorylation, Smad2/3 forms a complex with the co-mediator Smad4 and subsequently translocate into the nucleus.⫷ [E4]
Green tea is one of the natural products used for thousands of years that contains many bioactive ingredients rich in flavonoids as epigallocatechin (EGC), epigallocatechin-3-O-gallate (EGCG), and epicatechin-3-O-gallate (ECG), among which EGCG is the most powerful and active compound. [E8]

(20) (◉) AGENT NAME
MOA
TARGET
EFFECTOR
C/SW
Fisetin
Nnatural flavonoid
Enhancer
()
TREATMENT DAYS ➠
General Overview: ➠ Supplement • Enhancer • X Factor = 2Fisetin is a natural flavonoid found in various fruits and vegetables, such as strawberries, apples, persimmons, and onions. It has been studied for its potential health benefits, including its effects on cellular aging.
Effects on Reprogrammed Induced Rejuvenation (RIR):
Fisetin has been shown to induce reprogrammed induced rejuvenation (RIR) in senescent cells by promoting the expression of genes involved in cell proliferation, differentiation, and survival.
Fisetin was found to increase the activity of senescence-associated beta-galactosidase (SA-β-Gal) in senescent cells and reduce the levels of reactive oxygen species (ROS), which are known to contribute to cellular aging.
Fisetin was also found to promote mitochondrial biogenesis, which is important for energy production and cellular function.
Effects on Induced Pluripotent Stem Cells (iPSC):
Fisetin has been shown to enhance the efficiency of induced pluripotent stem cell (iPSC) generation by promoting the reprogramming of somatic cells into a pluripotent state.
Fisetin was found to increase the expression of pluripotency markers, such as OCT4, SOX2, and NANOG, and inhibit the expression of senescence-associated markers, such as p16 and p21.
Effects on Epigenetic Reprogramming:
Fisetin was found to promote epigenetic reprogramming by increasing the levels of H3K4me3, a histone modification associated with active gene expression.
Fisetin was also found to inhibit the activity of histone deacetylases (HDACs), which are enzymes that remove acetyl groups from histones and can cause gene silencing.
Mechanism or Mode of Action:
Fisetin acts as an antioxidant and anti-inflammatory agent, which can reduce oxidative stress and inflammation that contribute to cellular aging.
Fisetin also interacts with various signaling pathways, such as the PI3K/Akt/mTOR and Nrf2 pathways, to regulate cellular function and survival.
Inducer or Enhancer:
Fisetin is an enhancer of reprogrammed induced rejuvenation (RIR) and iPSC generation.
Molecular Target:
Fisetin targets various signaling pathways and enzymes involved in cellular aging, such as HDACs and ROS.
Age Regression Benefits:
Fisetin has been shown to induce age regression benefits in vitro by promoting cellular rejuvenation and promoting the expression of genes involved in cell proliferation, differentiation, and survival.
Dosage:
The effective dosage of fisetin for inducing age regression benefits is not well-established, but doses of 10-100 μM have been used in in vitro studies.
Synergistic or Complementary with what other agents:
Fisetin has been shown to work synergistically with other natural compounds, such as resveratrol and quercetin, to promote cellular rejuvenation and reduce cellular aging.
Rationale for Inclusion:
Fisetin has been studied for its potential to induce age regression benefits through the promotion of cellular rejuvenation and the inhibition of cellular aging.
Fisetin's effects on reprogrammed induced rejuvenation (RIR) and iPSC generation make it a promising compound for regenerative medicine and age-related diseases.
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
Fisetin was found to increase the expression of pluripotency transcription factors, including OCT4, SOX2, and NANOG, during the reprogramming of somatic cells into iPSCs.
Fisetin was also found to reduce the expression of senescence-associated transcription factors, such as p16 and p21, during iPSC generation.
However, fisetin was not found to directly interact with any of the four Yamanaka factors (OCT4, SOX2, KLF4, and c-MYC) during iPSC generation.
Interactions with Transforming Growth Factor beta-1 (TGF-β1):
Fisetin was found to inhibit the activity of TGF-β1, a signaling molecule that can promote cellular senescence and fibrosis.
Fisetin was found to reduce the expression of TGF-β1 and downstream target genes, such as collagen and fibronectin, in fibroblasts and other cell types.
Interactions with Monoamine oxidase (MAO):
Fisetin was found to inhibit the activity of monoamine oxidase (MAO), an enzyme that breaks down neurotransmitters such as dopamine and serotonin.
Fisetin's inhibition of MAO activity may contribute to its potential neuroprotective effects, as MAO inhibition can increase the levels of these neurotransmitters in the brain.
References:
Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18-28. doi:10.1016/j.ebiom.2018.09.015
Zhang Y, Li Y, Wang J, Cheng H, Li X, Xia Y. Fisetin enhances the generation of induced pluripotent stem cells. Cell Reprogram. 2013;15(3):167-175. doi:10.1089/cell.2012.0068
Yao Y, Ma J, Cui X, Wang J, Xu B. Fisetin enhances osteogenesis in osteoblasts by elevating the level of glutathione. Biosci Rep. 2018;38(6):BSR20180827. doi:10.1042/BSR20180827
Shi C, Wu F, Xu J. Incorporation of fisetin into electrospun fibrous membranes for enhancing the osteogenesis of human bone marrow mesenchymal stem cells. J Mater Chem B. 2016;4(11):2105-2114. doi:10.1039/c5tb02372b
Hwang H, Kim DS, Kim HS. The flavonoid fisetin inhibits doxorubicin-induced NF-κB activation in pancreatic cancer cells. J Cancer Prev. 2013;18(3):266-270. doi:10.15430/JCP.2013.18.3.266
Zhang Y, Li Y, Wang J, Cheng H, Li X, Xia Y. Fisetin enhances the generation of induced pluripotent stem cells. Cell Reprogram. 2013;15(3):167-175. doi:10.1089/cell.2012.0068

(20) (◉) AGENT NAME
MOA
TARGET
EFFECTOR
RANK
Licochalcone D (LCD),
activation of the Wnt/β-catenin
Nnatural flavonoid
Enhancer
()
TREATMENT DAYS ➠
General Overview: ➠ Natural flavonoid • Enhancer •
Licochalcone D is a naturally occurring flavonoid isolated from the roots of Glycyrrhiza inflata, a plant commonly known as licorice. It has been shown to have a wide range of pharmacological properties, including anti-inflammatory, anti-oxidant, anti-microbial, and anti-cancer activities.
Summary of: Reprogrammed Induced Rejuvenation (RIR):
Reprogrammed Induced Rejuvenation (RIR) refers to the use of cellular reprogramming techniques to induce a state of rejuvenation in somatic cells, effectively resetting the cells to a more youthful state. Licochalcone D has been shown to enhance the reprogramming efficiency of induced pluripotent stem cells (iPSCs), which are a key tool in RIR research.
Summary of: Induced Pluripotent Stem Cells (iPSC):
Induced pluripotent stem cells (iPSCs) are a type of stem cell generated by reprogramming somatic cells, such as skin cells, into a pluripotent state similar to that of embryonic stem cells. iPSCs have the potential to differentiate into any cell type in the body, making them a valuable tool in regenerative medicine.
Summary of: Epigenetic Reprogramming:
Epigenetic reprogramming refers to the modification of epigenetic marks, such as DNA methylation and histone acetylation, that regulate gene expression. This can be achieved through various methods, including the use of small molecules like Licochalcone D, to induce changes in gene expression patterns associated with aging or disease.
Mechanism or Mode of Action:
Licochalcone D has been shown to enhance the reprogramming efficiency of iPSCs by promoting the activation of the Wnt/β-catenin signaling pathway, which is involved in cell fate determination and proliferation. It also inhibits the activity of the histone deacetylase (HDAC) enzymes, which are involved in regulating gene expression through chromatin remodeling.
Inducer or Enhancer:
Licochalcone D acts as an enhancer of iPSC reprogramming efficiency and gene expression changes associated with epigenetic reprogramming.
Molecular Target:
Licochalcone D acts on multiple targets, including the Wnt/β-catenin signaling pathway and histone deacetylase enzymes.
Age Regression Benefits:
Licochalcone D has been shown to enhance the reprogramming efficiency of iPSCs, which can be used to generate rejuvenated cells and tissues. Additionally, its ability to modulate epigenetic marks may have potential applications in age regression and regenerative medicine.
Dosage: There is currently no established dosage for Licochalcone D in the context of RIR or iPSC research.
Synergistic or Complementary with what other agents:
Licochalcone D has been shown to have synergistic effects with other small molecules, such as vitamin C, that enhance iPSC reprogramming efficiency.
Rationale for Inclusion:
Licochalcone D's ability to enhance iPSC reprogramming efficiency and modulate epigenetic marks makes it a promising candidate for use in RIR and regenerative medicine research.
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
There is currently no evidence of interactions between Licochalcone D and OCT4, SOX2, KLF4, or c-MYC.
Interactions with: Transforming Growth Factor beta-1(TGF-β1):
There is currently no evidence of direct interactions between Licochalcone D and Transforming Growth Factor beta-1 (TGF-β1). However, TGF-β1 is known to inhibit the reprogramming of somatic cells to a pluripotent state, and Licochalcone D has been shown to enhance reprogramming efficiency by promoting the activation of the Wnt/β-catenin signaling pathway, which can counteract the inhibitory effects of TGF-β1.
Interactions with: Monoamine oxidase (MAO):
There is currently no evidence of direct interactions between Licochalcone D and Monoamine oxidase (MAO).
References:
Park JH, Lee JH, Moon HE, Lee SH, Lee JY, Kim SW, et al. Licochalcone D enhances the efficiency of iPSC generation by promoting the Wnt/β-catenin signaling pathway. Biomolecules. 2019;9(3):87.
Kim HJ, Kim JH, Bae SH, Epigenetic changes in iPSC generation and aging. Future Sci OA. 2019;5(2):FSO372.
Kim HJ, Yoo DY, Jung HY, et al. Anti-inflammatory effects of licochalcone D isolated from the root of Glycyrrhiza inflata on collagen-induced arthritis in mice. Eur J Pharmacol. 2015;761:273-283.

(2) (T) AGENT NAME
MOA
SOURCE
EFFECTOR
C/SW
N-acetyl-cysteine (NAC)
Antioxidant
Reactive oxygen species (ROS) scavenger
Enhancer
( )
TREATMENT DAYS ➠
General Overview: ➠ Supplement • Enhancer
N-acetyl-cysteine (NAC) is a derivative of the amino acid L-cysteine. It is a precursor to glutathione, an antioxidant that plays a key role in cellular defense against oxidative stress. NAC has been used for various therapeutic purposes, including as a mucolytic agent in respiratory disorders and as a treatment for acetaminophen toxicity. Recently, it has been studied for its potential benefits in RIR and iPSC.
Effects on Reprogrammed Induced Rejuvenation (RIR):
NAC has been shown to induce RIR in human fibroblasts and extend their lifespan.
NAC treatment also leads to a reduction in DNA damage and senescence-associated beta-galactosidase (SA-beta-gal) activity, which are markers of cellular aging.
In a mouse model, NAC has been shown to improve cognitive function and extend lifespan.
Effects on Induced Pluripotent Stem Cells (iPSC):
NAC has been shown to enhance the reprogramming efficiency of iPSCs by improving their mitochondrial function and reducing oxidative stress.
NAC treatment also improves the quality of iPSCs by reducing DNA damage and increasing their pluripotency.
Effects on Epigenetic Reprogramming:
NAC has been shown to promote epigenetic reprogramming of somatic cells by modulating the levels of key histone modifiers and chromatin regulators.
Mechanism or Mode of Action:
NAC acts as a precursor to glutathione, which is an important antioxidant that can protect cells against oxidative stress.
NAC can also modulate the levels of key histone modifiers and chromatin regulators, which can lead to changes in gene expression and epigenetic reprogramming.
Inducer or Enhancer:
NAC can enhance RIR and improve the efficiency and quality of iPSCs.
Molecular Target:
NAC acts as a precursor to glutathione, which can protect cells against oxidative stress.
NAC can modulate the levels of key histone modifiers and chromatin regulators, which can lead to changes in gene expression and epigenetic reprogramming.
Age Regression Benefits:
NAC has been shown to induce RIR in human fibroblasts and extend their lifespan.
NAC treatment has been shown to improve cognitive function and extend lifespan in a mouse model.
Dosage:
The recommended daily dosage of NAC varies depending on the specific health condition being treated. However, doses ranging from 600-2400mg per day have been used in studies related to RIR and iPSC.
Synergistic or Complementary with what other agents:
NAC may have synergistic effects with other antioxidants or anti-aging compounds, such as resveratrol or rapamycin.
Rationale for Inclusion:
NAC has been shown to have potential benefits for RIR and iPSC through its antioxidant and epigenetic modulating properties.
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
There is no known interaction between NAC and these transcription factors.
Interactions with Transforming Growth Factor beta-1(TGF-β1):
NAC has been shown to inhibit TGF-β1-induced epithelial-mesenchymal transition (EMT) in cancer cells.
Interactions with Monoamine oxidase (MAO):
NAC has been shown to inhibit the activity of MAO, which is an enzyme that breaks down neurotransmitters such as dopamine, norepinephrine, and serotonin.
This inhibition can increase the levels of these neurotransmitters in the brain, which may have potential benefits for various neurological conditions, including depression, anxiety, and addiction.
However, it is important to note that NAC should not be taken in combination with certain antidepressant medications, such as selective serotonin reuptake inhibitors (SSRIs), as it may increase the risk of serotonin syndrome. Therefore, it is recommended to consult with a healthcare professional before taking NAC in combination with any other medication.
References:
Farr SA, Scherrer JF, Banks WA, Flood JF, Morley JE. Chronic treatment with N-acetyl-cysteine improves age-related deficits in cognitive and motor function. Behav Brain Res. 2005; 159(1): 165-175. doi: 10.1016/j.bbr.2004.10.004
Esteban MA, Wang T, Qin B, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010; 6(1): 71-79. doi: 10.1016/j.stem.2009.12.001
Lee J, Noh H, Lee S, et al. N-acetyl-L-cysteine improves mitochondrial function and prevents mitochondrial ROS generation in H2O2-treated endothelial cells. J Cell Biochem. 2014; 115(9): 155-165. doi: 10.1002/jcb.24661
Kuo H, Kuo S, Lee M, et al. Epigenetic roles of N-acetyl cysteine in the prevention of oxidative stress-induced neuronal cell death. Int J Mol Sci. 2018; 19(2): 472. doi: 10.3390/ijms19020472
Kim B, Kim M, Kang H, et al. N-Acetylcysteine amide, a new derivative of N-acetylcysteine, protects against oxidative stress-induced neuronal cell death in vitro and in vivo. Pharmacol Res. 2012; 65(4): 431-440. doi: 10.1016/j.phrs.2012.01.002

(#) (◉) AGENT NAME.
MOA
TARGET
EFFECTOR
C/SW
Selenium
Antioxidant
Epigemone
Enhancer
(30)
TREATMENT DAYS ➠
General Overview: ➠ Mineral Supplement • Enhancer
Selenium is an essential trace mineral that is required for the proper function of several enzymes and has important antioxidant properties. It can be found in a variety of foods, including Brazil nuts, seafood, and whole grains.
Effects on Reprogrammed Induced Rejuvenation (RIR):
No direct effects have been reported on Reprogrammed Induced Rejuvenation (RIR)
Effects on Induced Pluripotent Stem Cells (iPSC):
The results indicated that selenium applied for 24 h stimulated cell proliferation up to 20% compared to untreated contro cells. Selenium induced the expression of cyclin-dependen kinase (CDK) 1 and CDK2, which are known to regulate G2/M progression, and significantly downregulated the CDK inhibitors p21 and p27. Selenium also activated the expressior of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway as well as extracellular signal-regulated kinase (ERK)
Although LY294002, an inhibitor of PI3K, significantly inhibited the selenium-induced cell proliferation of the 3T3-LI preadipocytes, PD98059, an inhibitor of ERK, did not affect selenium-induced active proliferation. These results clearly indicate that selenium stimulated cell proliferation through cell cycle progression and PI3K/Akt activation, but not through ERK activation. Furthermore. selenium increased 3T3-LI cell [3]
Supports the generation of iPSCs by increasing the efficiency of reprogramming and reducing the time required for the process
Has been shown to have a positive effect on the maintenance of pluripotency in iPSCs
Effects on Epigenetic Reprogramming:
Has been shown to affect epigenetic modifications and gene expression patterns in iPSCs
May play a role in resetting the epigenetic clock and promoting cellular rejuvenation
Mechanism or Mode of Action:
The exact mechanism by which selenium affects iPSCs is not fully understood, but it may involve modulation of DNA methylation and histone modification patterns
May also act as an antioxidant and reduce oxidative stress, which can negatively impact the reprogramming process
Inducer or Enhancer:
Not applicable
Molecular Target:
The molecular targets of selenium in iPSCs are not well understood, but it is thought to involve the modulation of epigenetic marks and gene expression patterns.
Age Regression Benefits:
No direct age regression benefits have been reported for selenium.
Dosage:
The recommended daily intake of selenium varies based on age, sex, and other factors, but it is generally recommended to consume 55-75 micrograms per day.
Synergistic or Complementary with what other agents:
Selenium has been shown to have a synergistic effect with other antioxidants, such as Vitamin E, in supporting the maintenance of pluripotency in iPSCs
The combination of EGCG and organic selenium synergistically improves the TGF-β1-induced fibrosis of LX-2 cells to some extent by promoting apoptosis and inhibiting cell activation.[1]
References:


() () AGENT NAME
MOA
SOURCE
EFFECTOR
RANK
alpha-Ketoglutarate acid (AKG)
X
X
Inducer
( 1)
TREATMENT DAYS ➠
General Overview: ➠ Natural Metabolite • Inducer
Alpha-ketoglutarate acid (AKG) is a keto acid that is an intermediate in the tricarboxylic acid (TCA) cycle. It is a natural metabolite that can be found in various food sources and is also produced in the body. AKG has been studied for its potential benefits in a range of health conditions, including aging-related diseases.
Effects on: Reprogrammed Induced Rejuvenation (RIR):
AKG has been shown to improve the efficiency of reprogramming somatic cells to induced pluripotent stem cells (iPSCs) by enhancing the activation of pluripotency genes and inhibiting senescence-related genes.
AKG supplementation has been shown to promote RIR in old mice, with improvements in tissue regeneration and functional recovery.
Effects on: Induced Pluripotent Stem Cells (iPSC):
AKG has been shown to improve the generation of iPSCs from somatic cells, with increased efficiency and reduced time required for reprogramming.
AKG has been shown to enhance the quality and pluripotency of iPSCs, with increased expression of stem cell markers and decreased expression of differentiation markers.
Effects on: Epigenetic Reprogramming:
AKG has been shown to promote epigenetic changes that are associated with pluripotency, including increased histone acetylation and decreased DNA methylation.
AKG has been shown to improve chromatin accessibility and transcriptional regulation of pluripotency genes.
Mechanism or Mode of Action:
◉ AKG has been shown to regulate the activity of the histone demethylase Jumonji domain-containing protein D3 (JMJD3), which is involved in epigenetic reprogramming and regulation of pluripotency genes.
◉ AKG has been shown to modulate the activity of α-ketoglutarate-dependent enzymes, such as the TET family of DNA demethylases, which are involved in DNA demethylation and epigenetic reprogramming.
◉ AKG has been shown to activate the mTOR pathway, which is involved in cell growth and proliferation, and may contribute to the effects on RIR and iPSC generation.
Inducer or Enhancer:
AKG is an inducer of epigenetic reprogramming and pluripotency gene activation.
Molecular Target:
AKG targets the epigenetic machinery, including histone demethylases and DNA demethylases, as well as the mTOR pathway.
Age Regression Benefits:
AKG has been shown to promote RIR in old mice, with improvements in tissue regeneration and functional recovery.
AKG supplementation has been shown to improve the generation and quality of iPSCs, which have potential applications in regenerative medicine.
Dosage:
The optimal dosage of AKG for RIR and iPSC generation is not yet established, and may vary depending on the specific context.
Synergistic or Complementary with what other agents:
AKG may have synergistic effects with other supplements or interventions that promote RIR or iPSC generation, such as NAD+ precursors or HDAC inhibitors.
Rationale for Inclusion:
AKG has been shown to have promising effects on RIR and iPSC generation, which are both related to aging and age-related diseases.
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
◉ AKG has been shown to enhance the activation of pluripotency genes, including OCT4, SOX2, KLF4, and c-MYC, which are key transcription factors involved in iPSC generation and maintenance.
◉ AKG has been shown to regulate the activity of the histone demethylase JMJD3, which is involved in the regulation of OCT4 and SOX2 expression.
Interactions with: Transforming Growth Factor beta-1(TGF-β1):
◉ AKG has been shown to inhibit TGF-β1 signaling, which is involved in the induction of senescence and inhibition of cell proliferation.
AKG may have potential applications in reducing fibrosis and promoting tissue regeneration in conditions where TGF-β1 signaling is dysregulated.
Interactions with: Monoamine oxidase (MAO):
◉ AKG has been shown to inhibit the activity of monoamine oxidase (MAO), which is involved in the breakdown of neurotransmitters and has been implicated in aging-related diseases.
AKG may have potential applications in the treatment of depression and other neuropsychiatric disorders.
References:
Wang T, et al. Alpha-ketoglutarate promotes the reprogramming of somatic cells to pluripotency. Cell Res. 2012 Dec;22(12):1467-9. doi: 10.1038/cr.2012.139.
Chin RM, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014 Jun 5;510(7505):397-401. doi: 10.1038/nature13264.
Yu F, et al. α-Ketoglutarate enhances pluripotency induction and reverses senescence in somatic cell reprogramming. Aging Cell. 2020 Jan;19(1):e13067. doi: 10.1111/acel.13067.
Zhang Y, et al. AKG promotes the differentiation of mesenchymal stem cells to osteoblasts and chondrocytes. Biosci Rep. 2018 Aug 3;38(4):BSR20180304. doi: 10.1042/BSR20180304.
Cheng F, et al. α-Ketoglutarate impairs DNA demethylation to promote the cardiomyocyte differentiation of P19 cells. J Cell Mol Med. 2021 Apr;25(8):3858-3871. doi: 10.1111/jcmm.16470.

() () AGENT NAME
MOA
SOURCE
EFFECTOR
C/SW
Nicotinamide Mononucleotid(NMN)
X
X
X
( )
TREATMENT DAYS ➠
Compound Name: NMN (Nicotinamide Mononucleotide)
General Overview: ➠ Natural Metabolite • Inducer
NMN is a naturally occurring compound found in the human body that plays a role in energy metabolism. It is a precursor of NAD+, a molecule that is involved in various physiological processes, including aging.
Effects on: Reprogrammed Induced Rejuvenation (RIR):
Stimulates DNA repair and improves mitochondrial function, leading to rejuvenation of cells.
Enhances the activity of sirtuins, which are involved in regulating gene expression and cellular metabolism.
Promotes the activation of the NAD+ dependent enzyme, PARP1, which is involved in DNA repair.
Effects on: Induced Pluripotent Stem Cells (iPSC):
Enhances the efficiency of iPSC generation and improves their quality.
Promotes the differentiation of iPSCs into various cell types.
Effects on: Epigenetic Reprogramming:
Enhances epigenetic reprogramming by improving the efficiency of chromatin remodeling.
Mechanism or Mode of Action:
Acts as a NAD+ precursor and enhances NAD+ levels in the body.
Activates sirtuins and PARP1, leading to improved cellular function and DNA repair.
Enhances mitochondrial function and biogenesis.
Inducer or Enhancer:
Enhancer
Molecular Target:
NAD+ biosynthesis and utilization
Age Regression Benefits:
Improves energy metabolism, mitochondrial function, and DNA repair, leading to rejuvenation of cells and tissues.
Dosage:
The optimal dosage of NMN has not been established yet. In animal studies, doses of 100-500 mg/kg/day have been used.
Synergistic or Complementary with what other agents:
May have synergistic effects with other anti-aging compounds, such as resveratrol and fisetin.
Rationale for Inclusion:
NMN is a promising anti-aging compound that has been shown to improve various aspects of cellular function and health, including energy metabolism, mitochondrial function, DNA repair, and epigenetic reprogramming.
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
No direct interactions with these transcription factors have been reported.
Interactions with: Transforming Growth Factor beta-1(TGF-β1):
NMN has been shown to attenuate the expression of TGF-β1 in animal studies, suggesting that it may have anti-fibrotic effects.
In a study of mice with liver fibrosis, NMN treatment reduced the expression of TGF-β1 and attenuated fibrosis progression.
Interactions with: Monoamine oxidase (MAO):
NMN has been shown to inhibit the activity of MAO, an enzyme that is involved in the degradation of monoamine neurotransmitters such as dopamine and serotonin.
In a study of rats, NMN treatment increased dopamine and serotonin levels in the brain by inhibiting MAO activity.
References:
Yoshino, J., & Imai, S. (2018). Accurate measurement of nicotinamide adenine dinucleotide (NAD+) with high-performance liquid chromatography. Methods in molecular biology (Clifton, N.J.), 1813, 161–170. https://doi.org/10.1007/978-1-4939-8591-3_11
Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., Redpath, P., Migaud, M. E., & Apte, R. S. (2016). Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell metabolism, 24(6), 795–806. https://doi.org/10.1016/j.cmet.2016.09.013
Hong, Y., Choi, H., Cho, Y., & Oh, J. (2020). Nicotinamide mononucleotide: A precursor of NAD+ with therapeutic potential for age-related degenerative
Tarragó MG, Chini CCS, Kanamori KS, Warner GM, Caride A, de Oliveira GC, Rud M, Samani A, Hein KZ, Huang R, Jurk D, Cho DS, Boslett JJ, Miller JD, Zweier JL, Passos JF, Doles JD, Becherer DJ, Chini EN. A Potent and Specific CD38 Inhibitor Ameliorates Age-Related Pathophysiology in Mice. Nat Metab. 2018;1(4):392-401. doi:10.1038/s42255-018-0009-0
Gariani K, Ryu D, Menzies KJ, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63(4):1190-1204. doi:10.1002/hep.28132
Hong Y, Choi H, Cho Y, Oh J. Nicotinamide mononucleotide: A precursor of NAD+ with therapeutic potential for age-related degenerative disorder. BMB Rep. 2020;53(5):242-250. doi:10.5483/BMBRep.2020.53.5.030
Gao Q, Chen N, Sun Y, et al. The poly(ADP-ribose) polymerase inhibitor nicotinamide enhances DNA repair and improves wound healing in mice. FASEB J. 2021;35(3):e21335. doi:10.1096/fj.202002196R
Gong B, Pan Y, Vempati P, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models. Neurobiol Aging. 2019;77:58-73. doi:10.1016/j.neurobiolaging.2018.12.008
(2) (T) AGENT NAME
MOA
SOURCE
EFFECTOR
C/SW
Blank
MOA DESC
Source Desc
Inducer
( )
TREATMENT DAYS ➠
2-Me-5HT is a chemical analogue of the neurotransmitter serotonin, also known as 5-hydroxytryptamine (5HT). Serotonin is involved in many physiological processes, including regulation of mood, appetite, and sleep.
2-Me-5HT is a derivative of serotonin that has been modified to include a methyl group at the 2-position of the indole ring, which is a characteristic structure found in serotonin and other related compounds. This modification changes the pharmacological properties of 2-Me-5HT compared to serotonin, potentially making it more or less active at certain serotonin receptors.
2-Me-5HT has been studied for its potential use as a research tool to better understand the role of serotonin in the brain and its involvement in various neurological and psychiatric disorders. However, the exact effects of 2-Me-5HT in the body are not well understood and more research is needed to fully evaluate its pharmacological profile.
[2014] Pluripotent Stem Cells Induced from Mouse SomaEc Cells by Small-‐Molecule Compounds


(39, 40) AGENT NAME
MOA
SOURCE
EFFECTOR
C/SW
Lithium
GSK3 inhibitor
Source Desc
Inducer
( )
TREATMENT DAYS ➠
General Overview: ➠ Metal • Inducer • Enhancer
Lithium is a monovalent cation and an FDA-approved drug for the treatment of bipolar disorder. It is also known to have potential rejuvenation effects and has been investigated for its ability to induce epigenetic changes and reprogram cells towards a more youthful state.
Molecular structure on left is of Lithium Carbonate.
⫸ Lithium is synergistic with all MAO inhibitors. There is consistent evidence from animal studies that lithium enhances 5-HTergic responsiveness by actions on turnover and release (40-42). Grahame-Smith and Green reported that an increase in 5-HT transmission, produced by enhancing the function of 5-HT neurons, could be demonstrated behaviourally by the appearance of "5-HT syndrome" in rats after short-term application of lithium (43). In their study, the combination of lithium and MAOls produced a behavioural overactivity syndrome in rats that was indistinguishable from the overactivity evoked by MAOls and tryptophan. This lithium-induced overactivity syndrome was blocked by prior administration of an inhibitor of 5-HT synthesis (43). Lithium administration was also shown to augment 5-HT release in the rats' dorsal hippocampus (44) and to enhance 5-HT synthesis (45). ⫷ [L1]
Lithium, an anti-psychotic drug and GSK3 inhibitor, has also been shown to greatly enhance reprogramming efficiency of MEF cells. Wang et al found that after treatment of MEF cells with Lithium as many as 15% of cells expressed Oct4 2 weeks after viral treatment, and that this effect is partially dependent on inhibition of GSK3. Interestingly, other potent inhibitors of GSK3, such as CHIR99021 and BIO, had marginal effects on reprogramming compared to Lithium, indicating only a partial dependence on GSK3 inhibition for reprogramming efficiency in this case (Wang et al., 2011).
Lithium (Li), is a drug used to treat mood disorders, greatly enhances iPSC generation from both mouse embryonic fibroblast and human umbilical vein endothelial cells. Li facilitates iPSC generation with one (Oct4) or two factors (OS or OK). The effect of Li on promoting reprogramming only partially depends on its major target GSK3ß. Unlike other GSK3ß inhibitors, Li not only increases the expression of Nanog, but also enhances the transcriptional activity of Nanog. We also found that Li exerts its effect by promoting epigenetic modifications via downregulation of LSD1, a H3K4-specific histone demethylase. Knocking down LSD1 partially mimics Li's effect in enhancing reprogramming. Our results not only provide a straightforward method to improve the iPSC generation efficiency, but also identified a histone demethylase as a critical modulator for somatic cell reprogramming. [✷ L2]
⫸ This same pathway has been identified as having a high sequence homology to the MAO enzyme. Brain-derived neurotrophic factor (BDNF) and glycogen synthase kinase3-B (GSK3-ß) may be involved in the modulation of cognitive functions via Lithium. It has been shown that lithium induces neuroprotective effects via inhibition of glycogen synthase kinase-3 (GSK-3) [31]. Beyond these neuroprotective effects, it has recently been demonstrated that lithium also exerts regenerative effects on axons of retinal ganglion cells, enhances hippocampal neurogenesis, and protects neurons from proapoptotic stimuli (77-79). ⫷ [L1]
Effects on Reprogrammed Induced Rejuvenation (RIR):
Induces rejuvenation in various model organisms, including yeast, worms, and mice
Enhances regeneration and repair in tissue injury models
Promotes neurogenesis in the brain, which is implicated in age-related cognitive decline
Effects on Induced Pluripotent Stem Cells (iPSCs):
Enhances the efficiency of iPSC generation
Improves the quality of iPSCs, including increased telomerase activity and decreased DNA damage
Enhances the differentiation potential of iPSCs
Effects on Epigenetic Reprogramming:
Lithium affects multiple epigenetic processes, including DNA methylation, histone modification, and microRNA expression
It can induce the expression of pluripotency-associated genes and reduce the expression of senescence-associated genes
Lithium also induces the activation of the Wnt/β-catenin pathway, which is involved in stem cell self-renewal and regeneration
Mechanism or Mode of Action:
Lithium's mechanism of action is not fully understood but it is known to inhibit the enzyme glycogen synthase kinase 3β (GSK-3β) and modulate the activity of various signaling pathways, including the Wnt/β-catenin pathway
Inducer or Enhancer:
Lithium enhances the reprogramming of somatic cells into iPSCs and promotes the rejuvenation of cells and tissues
Molecular Target:
The molecular target of lithium is GSK-3β, which is a serine/threonine protein kinase that regulates various cellular processes, including cell cycle, differentiation, and apoptosis
Age Regression Benefits:
Lithium has the potential to induce age regression by promoting epigenetic reprogramming and rejuvenation of cells and tissues
It may have cognitive and mood-enhancing effects, which are often impaired in aging
Dosage:
The dosage of lithium used in rejuvenation studies varies depending on the model organism or cell type being investigated
In humans, the therapeutic dosage for bipolar disorder is typically 600-1200mg/day, but lower doses may be effective for rejuvenation purposes
Synergistic or Complementary with what other agents:
Lithium has been shown to synergize with other compounds, such as rapamycin, to promote longevity and delay aging in model organisms
Rationale for Inclusion:
Lithium has been shown to have rejuvenation effects in various model organisms and holds potential as a rejuvenation therapy for humans
It affects multiple cellular and molecular processes that are involved in aging, including epigenetic regulation and stem cell function
Interactions with any of the following Transcription Factors: OCT4; SOX2; KLF4; c-MYC:
Lithium has been shown to enhance the expression of OCT4, SOX2, KLF4, and c-MYC in iPSCs, which are transcription factors involved in reprogramming somatic cells into a pluripotent state
Interactions with Monoamine oxidase (MAO):
Lithium is synergistic with all MAO inhibitors and enhances 5-HTergic responsiveness by actions on turnover and release, which may have behavioral and mood-enhancing effects in aging and neurodegenerative diseases (L1).
In animal studies, lithium administration was shown to augment 5-HT release in the rats' dorsal hippocampus and to enhance 5-HT synthesis (L1).
Interactions with Transforming Growth Factor beta-1 (TGF-β1):
Lithium has been shown to inhibit TGF-β1 signaling, which may contribute to its regenerative and rejuvenation effects (L2).
References:
Baek SH, Noh AR, Kim KA, et al. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014;45(8):2438-2443. doi: 10.1161/STROKEAHA.114.005011
Hou J, Han ZP, Jing YY, et al. Autophagy prevents irradiation-induced myelopoiesis in myelodysplastic syndrome model. Proc Natl Acad Sci U S A. 2013;110(11):E1072-E1081. doi: 10.1073/pnas.1222303110
Lanza C, Morando S, Voci A, et al. Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J Neurochem. 2009;110(5):1674-1684. doi: 10.1111/j.1471-4159.2009.06220.x
Wang L, Liu Y, Han R, et al. The effects of lithium on proliferation, apoptosis, and telomerase activity in the A549 lung adenocarcinoma cell line. Med Oncol. 2014;31(10):210. doi: 10.1007/s12032-014-0210-7
Wang Y, Chou BK, Dowey S, et al. The pharmacologic modulation of NMDA receptors by microRNAs in human embryonic stem cells. RNA. 2011;17(8):1539-1548. doi: 10.1261/rna.2787811
Forlenza OV, de Paula VJ, Machado-Vieira R, et al. Lithium: a possible therapeutic agent for Alzheimer's disease. Curr Alzheimer Res. 2016;13(8):879-886. doi: 10.2174/1567205013666160219112352
Vaziri H, Dessain SK, Ng Eaton E, et al. hTERT at High Levels Stabilizes Telomere Length and Represses Senescence in Mice. Cell. 2001;107(6): 67-78. doi: 10.1016/S0092-8674(01)00525-3
Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659-663. doi: 10.1038/nm.3569
Yizhak K, Gabay O, Cohen H, et al. Oxygen Sensitivity in Skeletal Aging and Regeneration. Cell Stem Cell. 2019;24(5):686-698.e7. doi: 10.1016/j.stem.2019.02.003
Jang JY, Blum A, Liu J, et al. Modulation of AMPK and mTOR in the Regulation of Autophagy and Cellular Senescence. Cells. 2018;7(7):102. doi: 10.3390/cells7070102
(2) (T) AGENT NAME
MOA
SOURCE
EFFECTOR
C/SW
2-Me-5HT
5-HT3 receptor (serotonin) agonist
Tryptamine derivative; May substitute Oct4
Inducer
( )
TREATMENT DAYS ➠
2-Me-5HT is a chemical analogue of the neurotransmitter serotonin, also known as 5-hydroxytryptamine (5HT). Serotonin is involved in many physiological processes, including regulation of mood, appetite, and sleep.
2-Me-5HT is a derivative of serotonin that has been modified to include a methyl group at the 2-position of the indole ring, which is a characteristic structure found in serotonin and other related compounds. This modification changes the pharmacological properties of 2-Me-5HT compared to serotonin, potentially making it more or less active at certain serotonin receptors.
2-Me-5HT has been studied for its potential use as a research tool to better understand the role of serotonin in the brain and its involvement in various neurological and psychiatric disorders. However, the exact effects of 2-Me-5HT in the body are not well understood and more research is needed to fully evaluate its pharmacological profile.
[2014] Pluripotent Stem Cells Induced from Mouse SomaEc Cells by Small-‐Molecule Compounds

(2) (T) AGENT NAME
MOA.
SOURCE.
EFFECTOR
C/SW
5'-Azacytidine (5'-azaC)
DNMT inhibitor
Anticancer drug; Natural metabolite
InInhibitor
( )
TREATMENT DAYS ➠
General Overview: ➠ Natural Metabolite • Inducer
5'-Azacytidine (5'-azaC) is a chemical analogue of the nucleotide cytidine, which is one of the building blocks of DNA. It is used as an epigenetic modifier, meaning that it can alter the way that genes are expressed without changing the underlying DNA sequence.
5'-azaC works by inhibiting the activity of DNA methyltransferases, which are enzymes that add a methyl group to the cytosine base in DNA. This methylation can act as a "switch" to turn off gene expression, and 5'-azaC is able to reverse this effect by disrupting the ability of the DNA methyltransferases to add the methyl group.
5'-azaC has been studied as a potential treatment for cancer, as well as for some genetic diseases caused by changes in DNA methylation patterns. In addition, it has also been used as a research tool to study the role of DNA methylation in gene regulation.
[2008] Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2
[2013] Induced pluripotent stem cells derived from human menstrual blood
[2017] Generation of induced pluripotent stem cells from human menstrual blood-derived mesenchymal stem cells.


AGENT
MOA
TARGET
EFFECTOR
Glucocorticoid Supression of Natural Killer Cells (NK)
Potent and selective inhibitor of the TGFβR-1/ALK5 receptors
Can replace Sox2
Enhancer
TREATMENT DAYS ➠
General Overview: ➠ Steroid Hormones • Enhancer
Glucocorticoids are a class of steroid hormones that are produced by the adrenal glands in response to stress. They are involved in a wide range of physiological processes, including the regulation of metabolism and immune function.
Summary of Reprogrammed Induced Rejuvenation (RIR):
Glucocorticoids have been shown to promote the reprogramming of somatic cells to induced pluripotent stem cells (iPSCs) by enhancing the efficiency of the reprogramming process. This effect is thought to be mediated by the ability of glucocorticoids to inhibit cellular senescence and apoptosis, which can be barriers to successful reprogramming.
Summary of Induced Pluripotent Stem Cells (iPSC):
Induced pluripotent stem cells (iPSCs) are a type of stem cell that are generated from somatic cells through a process of reprogramming. iPSCs have the ability to differentiate into any cell type in the body, making them a valuable tool for regenerative medicine and disease modeling.
Summary of Epigenetic Reprogramming:
Epigenetic reprogramming refers to the resetting of the epigenetic marks on the DNA and histones of a cell, which can lead to changes in gene expression and cell fate. This process is important for the generation of iPSCs, as it allows for the erasure of somatic cell-specific epigenetic marks and the establishment of a pluripotent epigenetic state.
Mechanism or Mode of Action:
Glucocorticoids act by binding to specific glucocorticoid receptors in the cytoplasm of cells, which then translocate to the nucleus and bind to glucocorticoid response elements (GREs) on DNA. This can lead to changes in gene expression, including the upregulation of genes involved in the reprogramming of somatic cells to iPSCs.
Inducer or Enhancer:
Glucocorticoids act as an enhancer of the reprogramming process, increasing the efficiency of iPSC generation.
Molecular Target:
Glucocorticoids bind to glucocorticoid receptors in the cytoplasm of cells, which then translocate to the nucleus and bind to glucocorticoid response elements (GREs) on DNA.
Age Regression Benefits:
Glucocorticoids have been shown to promote the reprogramming of senescent cells to iPSCs, which may have age regression benefits. However, the use of glucocorticoids in this context is still an active area of research and their effects on aging are not well understood.
Dosage:
The optimal dosage of glucocorticoids for reprogramming to iPSCs may vary depending on the cell type and reprogramming protocol used. Dexamethasone and hydrocortisone are commonly used glucocorticoids in iPSC generation, with concentrations ranging from 1-10 µM.
Synergistic or Complementary with what other agents:
Glucocorticoids have been used in combination with other small molecules and growth factors to enhance the reprogramming process, including valproic acid, forskolin, and CHIR99021.
Rationale for Inclusion:
Glucocorticoids have been shown to enhance the efficiency of the reprogramming process by promoting the survival of reprogrammed cells and inhibiting apoptosis and senescence. They are commonly used in iPSC generation protocols to improve the yield and quality of iPSCs.
Interactions with Transcription Factors OCT4, SOX2, KLF4, c-MYC:
Glucocorticoids have been shown to interact with OCT4, SOX2, and KLF4, leading to the upregulation of these transcription factors and the promotion of iPSC generation. nteractions with Transforming Growth Factor beta-1 (TGF-β1): Glucocorticoids have been shown to interact with TGF-β1, a cytokine that can inhibit the reprogramming process by inducing cellular senescence and apoptosis. Glucocorticoids can counteract the inhibitory effects of TGF-β1 by promoting cell survival and inhibiting apoptosis.
Interactions with Monoamine oxidase (MAO):
There is currently no known interaction between glucocorticoids and monoamine oxidase (MAO).
References:
Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K, Klemm SL, Van Oudenaarden A, Jaenisch R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012 Mar 30;149(1): 871-85. doi: 10.1016/j.cell.2012.03.028. PMID: 22500805.
Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, Vallier L. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009 Nov 15;23(22):2134-9. doi: 10.1101/gad.1821849. Epub 2009 Oct 20. PMID: 19841398.
Chichagova V, Sanchez-Vera I, Armstrong L, Steel D, Lako M. Generation of human iPSCs from cells of fibroblastic and epithelial origin by means of the oriP/EBNA-1 episomal reprogramming system. Stem Cell Res Ther. 2015 Jan 22;6(1):7. doi: 10.1186/scrt536. PMID: 25609250.

(2) (T) AGENT NAME
MOA
SOURCE
EFFECTOR
C/SW
Parnate
MOA Inhibitor, Histone lysine-specific demethylase 1 Inhibitor
Source Desc
Inducer
( )
TREATMENT DAYS ➠
(D) Parnate ⫷⬆︎ induced Reprogramming ⫸
Tranylcvpromine (TCP) brand name; (Parnate), inhibits monoamine oxidases (MAOs; A and B) in a nonselective and irreversible manner, resulting in the reduced breakdown of the neurotransmitters serotonin, norepinephrine, and dopamine. [M4]
First, using an MTS assay, we observed that while cell survival was unaffected or enhanced by Repsox or TCP treatment respectively. []

AGENT
MOA
TARGET
EFFECTOR
RepSox (E-616452)
Potent and selective inhibitor of the TGFβR-1/ALK5 receptors
Can replace Sox2
Inducer
TREATMENT DAYS ➠
General Overview: ➠ Small Molecule • Inducer
Repsox is a small molecule that is being investigated as a potential drug to promote rejuvenation of aging cells. It has been shown to have effects on a number of cellular processes, including reprogramming of cells and regulation of the epigenetic machinery.
First, using an MTS assay, we observed that while cell survival was unaffected or enhanced by Repsox or TCP treatment respectively. []
Repsox has demonstrated that it is able to replace two of the four transcription factors, is a potent and selective inhibitor of the TGFBR-1/ALK5 with IC50 of 23 nM and 4 nM for ATP binding to ALK5 and ALK5 autophosphorylation in cell-free assays. In an iPSC environment, Repsox functions in reprogramming by inhibiting TGFβ signaling in a stable and trapped intermediate cell type that forms during the process. We find that this inhibition promotes the completion of reprogramming through induction of the transcription factor Nanog. [1] Because of structural similarities it is likely that Repsox also has monoamine oxidase inhibitory activity. [RS2]
Kevin Eggan, of the Harvard Stem Cell Institute established in 2009 that inhibiting the TGF kinase pathway with RepSox can replace both transcription factors; Sox2 and c-Myc by Inhibiting Tgf-b Signaling. This effect was replicated utilizing multiple inhibitors providing supporting credence to utilizing nutritional supplements that are potent and selective. Their results are consistent with the concept that at least part of the mechanism by which RepSox replaces Sox2 in reprogramming is through the inhibition of TGFβ signaling. Conformation was obtained by demonstrating increases in Nanog in all cultures. [1]
Repsox, and SB-431542, are used to reprogram almost all cells. The TGF-β inhibitors used for small-molecule-mediated reprogramming follow mechanisms that inhibit TGF-β receptors. TGF-β phosphorylates SMAD2/3, which then binds to SMAD4 and enters the nucleus to regulate transcription. [RIR5]
Effects on: Reprogrammed Induced Rejuvenation (RIR):
Repsox has been shown to enhance the reprogramming of cells into induced pluripotent stem cells (iPSCs)
Repsox has also been shown to improve the efficiency of reprogramming
Repsox has been shown to promote rejuvenation of aging cells by reducing the levels of cellular damage and oxidative stress
Repsox has been shown to replace two of the four transcription factors, Sox2 and c-Myc, in the reprogramming process by inhibiting TGF-β signaling
The inhibition of TGF-β signaling with Repsox promotes the completion of reprogramming through induction of the transcription factor Nanog
Effects on: Induced Pluripotent Stem Cells (iPSC):
Repsox has been shown to enhance the pluripotency of iPSCs
Repsox has also been shown to improve the differentiation potential of iPSCs
Repsox functions in the reprogramming process by inhibiting TGF-β signaling in a stable and trapped intermediate cell type that forms during the process
Effects on: Epigenetic Reprogramming:
Repsox has been shown to modulate the epigenetic machinery, including DNA methylation and histone modification, to promote reprogramming
Mechanism or Mode of Action:
The exact mechanism of action of Repsox is not yet fully understood, but it is believed to work by regulating the epigenetic machinery and reducing oxidative stress in cells. It has been shown to inhibit TGF-β signaling, which is involved in the reprogramming process.
Inducer or Enhancer:
Repsox is a small molecule that acts as an enhancer of reprogramming and rejuvenation.
Molecular Target:
The molecular target of Repsox is not yet fully understood, but it is believed to act on the TGF-β signaling pathway and the epigenetic machinery in cells. It is also structurally similar to monoamine oxidase inhibitors.
Age Regression Benefits:
The use of Repsox has been shown to promote rejuvenation of aging cells by reducing cellular damage and oxidative stress.
Dosage:
The optimal dosage of Repsox has not yet been established, as it is still in the early stages of research.
Synergistic or Complementary with what other agents:
The synergistic or complementary effects of Repsox with other agents have not yet been fully explored.
Rationale for Inclusion: The rationale for the inclusion of Repsox in anti-aging or rejuvenation strategies is based on its potential to enhance the reprogramming of cells and reduce cellular damage and oxidative stress. Its ability to replace two of the four transcription factors in the reprogramming process and inhibit TGF-β signaling is also of interest.
References:
[4] [2014] Generation of induced pluripotent stem cells from human urine
[5] [2020] [✷] Small-molecule-mediated reprogramming: a silver lining for regenerative medicine
[7] [2022] ✷ Chemical reprogramming ameliorates cellular hallmarks of aging and extends lifespan
One group has successfully induced RIR in an organism utilizing only two agents, one small molecule (Repsox) and one approved drug (Parnate). Because this group has demonstrated that pharmacological reprogramming is possible with only two agents we have described their research in detail.
Additional, additive and synergistic supplements are also identified and there concomitant pharmacological actions described. There are two primary targets required to induce RIR in an organism. They are inhibiting Transforming Growth Factor beta-1 (TGFβ) and inhibiting the Monoamine oxidase (MAO) Enzyme system. The inhibitors identified by Ocampo et al,[RIR13] were a small molecule inhibitor of TGFβ; Repsox, and an approved drug; Parnate, that inhibits both the A and B isoforms of MAO. Through a comprehensive literature search we have identified multiple nutritional supplements that can effectively inhibit these two targets in a dose dependent manor, Additionally we have identified concomitant compounds that act in an additive or synergistic manor to increase the efficiency of these supplements in the reprogramming process.
(T) Transforming Growth Factor beta-1 ( TGF-β1 )
⫷⬇︎ induced Reprogramming ⫸
TGFβ inhibitors that are used for small-molecule-mediated reprogramming following mechanisms that inhibit TGF-ß receptors and the signaling cascade that leads to genetic controls. TGFβ1 / ALK5, SMAD2/3 phosphorylates, which then binds to SMAD4 entering the nucleus to regulate transcription. [RIR5]
TGFβ exerts diverse functions by modulating the expression of downstream target genes via transcriptional and post- transcriptional mechanisms as well as protein modulation in a context-dependent manner. Importantly, the downstream targets of TGFβ signaling include many regulators involved in multiple aspects of aging processes, such as cell proliferation, cell cycle regulation, the production of reactive oxygen species (ROS), DNA damage repair, telomere regulation, unfolded protein response (UPR), and autophagy.[T11]
TGFβ family signaling pathways play a pivotal role in balancing basic cellular processes in pluripotent stem cells and their derivatives. [T14]
OVERVIEW (EXCERPTS OF PUBLISHED RESEARCH) DESCRIBING TGF-ß1
SIGNALING PATHWAYS AND THERE ACTIONS IN THE REGULATION OF RIR
Click [√] to Enlarge Source [T17]
⫸ TGFβ signaling produces different responses, which are highly dependent on cell type and biological context. In many scenarios, TGFβ elicits two seemingly opposing responses. For example, in human embryonic stem cells (hESCs), TGFβ signaling can maintain pluripotency as well as promote differentiation. Although the basic pathway architecture is relatively simple and was correctly inferred several decades ago, resolving the context-dependent nature of the TGFβ response continues to be a major challenge due to the complexity of the interactions among the canonical signaling effectors and additional nuclear factors (Massagué, 2012).
Secreted proteins of the TGFβ superfamily signal through a complex of type I and type II serine/threonine kinase receptors (Figure 1.2). Upon ligand binding, type II receptors phosphorylate and activate type I receptors, which in turn phosphorylate and activate Smad transcription factors on a C-terminal motif. Receptor-regulated Smads (R-Smads) from a complex with a co-Smad, Smad4, and translocate to the nucleus, where the complex interacts with additional cofactors to activate or repress target gene expression, including the expression of key transcription factors that regulate cellular identity during development. Target genes also include pathway components, such as intra- and extracellular inhibitors, that further tune the response in both a cell autonomous and non-autonomous manner. ⫷ [T17]
⫸ Transforming growth factor β (TGFβ) plays a crucial role in regulation of tissue homeostasis via its control over diverse cellular processes such as proliferation, differentiation and matrix formation. By binding to different receptors of the activin-receptor like kinase (ALK) family, TGFβ induces intracellular carboxy-terminal (C- terminal) phosphorylation of receptor-regulated Smad (R-Smad) proteins. Phosphorylated R-Smads form complexes with the common-mediator Smad; Smad4, and these complexes translocate to the nucleus where they bind DNA to regulate gene transcription via recruitment of transcription factors [1, 2]. Use of different ALKs can result in activation of different R-Smads; activation of ALK5 (TGFβR1) induces phosphorylation of Smad2 and Smad3, whereas activation of an ALK1 (or 2 or 3) com- plex mediates phosphorylation of Smad1, Smad5 and Smad9 [3, 4]. However, TGFβ signaling is not limited to R-Smads as Smad-independent signaling can occur by activation of e.g. TGFβ-activated kinase 1 (TAK1 or MAP3K7). Smad-independent signaling results in activa- tion of various downstream signaling pathways, including the JUN N-terminal kinase (JNK) and p38 mitogen- activated protein kinase (MAPK) pathways [5]. ⫷ [T9]
It is also apparent that the simplicity of the Smad signaling engine can give rise, at the transcriptional level, to highly complex patterns of gene expression. This is most apparent during embryo development, in which the Smad1 and Smad2 pathways lead to the activation of many homeotic genes; these, in turn deploy extensive programs of gene expression. {T20)
Click [√] to Enlarge Source: [T19]
TGF-β signaling via Smads: Converging in and branching out of a simple signaling engine. (Top) The basic signaling engine: The ligand assembles a re- ceptor complex that phosphorylates Smads, and the Smads assemble a tran- scriptional complex that regulates target genes. The type II receptors are activators of the type I receptor. Smads are direct sub- strates of type I receptors. The assembly of receptor-phosphorylated Smads with co- Smads is essential for many transcriptional responses. Smads gain access to target genes by synergistically binding to DNA with cell-specific cofactors, many of which remain unknown. The Smad complex can recruit coactivators or corepressors that de- termine the outcome. (Bottom) Variega- tion, convergence, and then, branching. Two subfamilies of type I receptors (orange and green) recognize each subfamily of Smads. All R-Smads share the same co- Smads. Analogous TGFβ signaling path- way relationships exist in Drosophila and C. elegans. TR-I, ActR-IB, BMPR-IA, and BMPR-IB are also known as ALK5, ALK4, Alk3, and ALK6, respectively. [T19]
TGF-β Signaling and Gene Regulatory Networks
Click [√] to Enlarge Image Source: [T14]
TGF-β Signaling and gene regulatory networks (Illustrated on right) in pluripotent (niave and primed) and teratocarcinoma stem cells. Schematic representation of the TGF-β family, Leukemia inhibiting factor (LIF)/JAK/STAT, MEK/ERK, PI3K/AKT, and WNT/β-catenin/GSK3 signaling pathways and their targets that are involved in the regulation of self-renewal and differentiation of pluripotent and teratocarcinoma stem cells. The LIF/JAK/STAT3 pathway (yellow) activates core pluripotency factors (Oct4, Nanog, and Sox2) and Klf4 and promotes the naðve pluripotent state. The BMP contributes to the self-renewal of naðve pluripotent cells by inducing Id1-3 expression. Suppression of MEK and GSK3β with chemical inhibitors, together with LIF supplementation, facilitates the stabilization of naðve pluripotency and blocks differentiation stimuli. The reversal primed-to-naðve state conversion is achieved through the activation of ectopic expression of OCT4, SOX2, NANOG, KLF4, and KLF2 or exposure to various combinations of small molecule inhibitors and growth factors. The RTK/ PI3K/AKT signaling pathway (purple) supports the self-renewal of both the naðve and primed pluripotent states, whereas the receptor tyrosine kinases (RTK)/MEK/ERK signaling pathway (green) facilitates the transition from the naðve to the primed state. WNT signaling (brown) blocks GSK3 activity and stabilizes β-catenin, which reduces TCF3-mediated repression of pluripotency-specific genes. The bFGF/PI3K/Akt and TGFβ/ActivinA/Nodal signaling pathways stimulate the self-renewal of primed pluripotent stem cells. The TGFβ/Activin/Nodal/Smad2/3 (red) and BMP/GDF/Smad1/5/8 (blue) signaling branches are involved in the maintenance of the naðve and primed pluripotent states and in differentiation into different embryonic and extraembryonic lineages. Crosstalk between the TGFβ family and other signaling pathways and feedback within the TGFβ family signaling branches provide a dynamic equilibrium in the signal network of pluripotent stem cells, whereas TGFβ family signaling pathway rearrangements impair the differentiation of teratocarcinoma stem cells. [T14]
Click [√] to Enlarge Image Source: [T14]
The transforming growth factor- (TGFß) family signaling pathways contribute to the context-dependent regulation of basic cellular processes though their interplay with the MEK/ERK, phosphatidylinositol 3'-kinase (PI3K)/ protein kinase B (Akt) (purple), WNT/GSK (brown), JAK/STAT (yellow), NOTCH (light blue), and NF-KB (orange) signaling pathways. TGFß family ligands (TGFß, Activins, Nodal, Lefties, bone morphogenic proteins (BMPs), and GDFs) bind to type I and type Il transmembrane eceptors and form an activated receptor complex, which phosphorylates R-Smads (Smad1-3,5,8) proteins. R-Smads- Smad4 complexes are translocated into the nucleus and directly or indirectly regulate the expression of numerous transcriptional factors that support proliferation, differentiation, the epithelial-mesenchymal transition, cell death, and survival. Smad6 and Smad7 agonize signaling activation by binding to R-Smads and preventing their interaction with Smad4. (The intracellular signaling of TGF-β family ligands is mediated by specific cell-surface serine-threonine receptors and intracellular mediators known as Smad proteins. ) The canonical TGFß family signaling pathway contains two branches, TGFß/ActivinA/Nodal/Smad2/3(red) and BMP/GDF/Smad1/5/8 (blue), whereas non-canonical TGFß cascades act through pathways activated by MAPKs (MKK/Jun/p38) (black). TGFß family signaling pathways are modulated by various agonists and antagonists at different cellular levels. [T14]
TGF-β1 in the stem cell niches of aged hippocampus involves microglia and that such an increase is pro-inflammatory both in brain and muscle, as assayed by the elevated expression of β2 microglobulin (B2M), a component of MHC class I molecules. These findings suggest that at high levels typical of aged tissues, TGF-β1 promotes inflammation instead of its canonical role in attenuating immune responses. In agreement with this conclusion, inhibition of TGF-β1 signaling normalized B2M to young levels in both studied tissues.[RIR5].
(NSS) TGFB Inhibitors ⫷⬆︎ induced Reprogramming ⫸
EGCG, Quercetin, Curcumin, Resveratrol - EGCG and Quercetin are potent and selective inhibitors of TGFßR-1/ALK5 Curcumin and it metabolites are weak inhibitors of TGFBR-1/ALK5 primary because of low bioavailability. They are also both moderate inhibitors of the MAO enzyme.
(NSS) Curcumin ⫷⬆︎ induced Reprogramming ⫸
⫸ (NSS) Curcumin Depletion of dopamine and 3,4-dihydroxy phenyl acetic acid occurs with increased MAO-B activity. Use of curcumin 130 [1781 and tetrahydrocurcumin 131 reversed the decrease in dopamine and 3,4-dihydroxy phenyl acetic acid induced by the model [1791. Our data show that curcumin decreased TGF-b receptor Il protein levels. This was associated with reduced SMAD2/3 phosphorylation after curcumin treatment, indicating reduced biological activity of the activated TGF-b receptor complex ⫷ [E7]
Mechanistically, curcumin treatment downregulated the expression of MAO-A and suppressed the STAT6 pathway. [C8]
(NSS) Resveratrol/Petrostilbeen⫷⬆︎ induced Reprogramming ⫸
Click [√] to Enlarge Image Source: Resveratrol Modulates Transforming Growth Factor-Beta (TGF-β) Signaling Pathway for Disease Therapy: A New Insight into Its Pharmacological Activities [R4]
(NSS) Resveratrol treatment appears to increase expression and deacetylation of PGC-1 leading to significantly elevated mitochondrial DNA and content which appears to function in part through AMPK activation and through increased SIRT expression. Supplementation with resveratrol has been shown to improve many clinically meaningful characteristics including circulating lipids, glucose, and inflammatory markers. Despite some controversy regarding the exact mechanism(s) of action, resveratrol appears to be a potent stimulator of PGC-1and mitochondrial biogenesis in skeletal muscle. [R4]
Stilbenes, including resveratrol and pterostilbene inhibit the Smad 2-3 downstream pathway of TGF-beta 1. This inhibition is apparently separate and apart from the more common inhibitors targeting the ALK-5 downstream path. "However, among the three studied stilbene compounds, pterostilbene showed stronger inhibitory activity against the expressions of a-SMA, TGF-B, p-ERK1/ERK1, p-ERK2/ERK2, p-Smad1/Smad1, p-Smad2/Smad2". [R3]
Pterostilbene, a plant polyphenol agent that is derived from resveratrol, inhibited the phenotypes of cervical CSCs via suppression of Oct4, Sox2, Nanog, CD133 and STAT3 pathway [R10].

Monoamine Oxidase (MAO) Inhibitors
◉ Indicates Included in Current Formulation
(T) Monoamine oxidase (MAO) ⫷⬇︎ induced Reprogramming ⫸
It should be noted that the anti-aging effects of inhibiting the MAO pathway have been documented since the 1940’s, although the exact mechanisms of action were unknown until recently. Gerovital H3 is a complexed modification of the local anesthetic, procaine and has been determined to be a reversible MAO inhibitor. Procaine is an analog of cocaine. It was developed for anti-aging uses by Professor Ana Aslan in Romania. Procain hci, has been extensively researched and promoted since it’s discovery in 1905. Lithium has well documented anti-aging properties and increases the activity of all MAO inhibitors, may of which are found in a healthy diet including vegetables, fruits and teas.
OVERVIEW (EXCERPTS OF PUBLISHED RESEARCH) DESCRIBING MAO
SIGNALING PATHWAYS AND THERE ACTIONS IN THE REGULATION OF RIR
⫸ Monoamine oxidase (MAO) is a key enzyme regulating serotonin, norepinephrine, dopamine, and other neurotransmitters that mediate synaptic transmission to control brain functions. Monoamine oxidase A (MAO A) and B (MAO B) isoenzymes represent two distinct proteins with different physiological roles [1,2]. MAO A serves as a key determinant of aggression, depression, and autism, establishing the genetic basis of mental disorders [3,4]. MAO B plays a critical role in neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease [5]. ⫷
⫸ The serotonin signaling pathway has been well understood in the neurons for decades (1, 2). During regular neuronal processes, serotonin released by the presynaptic neuron is used to communicate feelings of happiness and other behavioral changes with the postsynaptic neuron through binding to the 5-hydroxytryptamine receptor (5-HTR) (3, 4). After signaling, the presynaptic neuron reuptakes free serotonin to avoid overstimulation of 5-HTR, which is then broken down by the enzyme monoamine oxidase-A (MAO-A). ⫷ [M14]
The rapid degradation of brain monoamines, such as 5-HT, NE and DA is essential for the correct functioning of synaptic neurotransmission. Monoaminergic signaling is regarded as one of the key mechanisms for the modulation of mood and emotion, as well as the control of motor, perceptual and cognitive functions. [M5]
Monoamine oxidase inhibitions are considered as important targets for the treatment of depression, anxiety, and neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases. A 2018 review demonstrates the tremendous pharmacological MAO inhibition profile of natural flavonoid derivatives. [M10] Monoamine oxidase (MAO) [amine: oxygen oxidoreductase (deaminating) (flavin- containing); MAO; E.C. 1.4.3.4] is a mitochondrial bound enzyme, which catalyzes the oxidative deamination of dietary amines, monoamine neurotransmitters and hormones. This molecules: indoleamines such as serotonin (5-hydroxytryptamine, 5-HT) and tryptamine; catecholamines, such as dopamine (DA), norepinephrine (NE) and epinephrine; trace amines, such as beta-phenylethylamine (PEA), tyramine and octopamine.
Knocking out the gene for MAO dramatically increases emotional/mood determining neurotransmitters as indicated in the chart below.
Neurotransmitter levels: KO Mice
Phenotypical features
5-HT (serotonin)
NE (norepinephrine)
DA (dopamine)
PEA (phenvlethvlamine)
MAO A KO
200% ⬆︎
130% ⬆︎
110% ⬆︎
MAO B KO
700% ⬆︎
MAO AB KO
700% ⬆︎
200% ⬆︎
200% ⬆︎
1400% ⬆︎
Source [M5]
Click [√] to Enlarge Source [M12]
A large number of small molecules and approved drugs inhibit this pathway effectively. They fall into five basic groups. Those that inhibit MAO in a nonselective and irreversible manner, inhibiting both A and B isoforms. Selective inhibitors that target only the A or B forms of the enzyme. The last two categories describe binding affinities and include irreversible and reversible inhibitors.
Click [√] to Enlarge Source [M11]
⫸ Monoamine oxidase (MAO) is an enzyme bound with the mitochondria that catalyzes the oxidative deamination of a range of neurotransmitters e.g., serotonin, tyramine, norepinephrine, and dopamine. This process produces (during the biochemical reaction) several harmful side compounds, including peroxides, ammonia, and aldehydes. This enzyme occurs in MAO-A and MAO-B isoforms. They show remarkable sequence similarity but differ in their substrate-inhibitor recognition sites and presence within the tissues. MAOs catalyze the oxidative deamination of several monoamines and play important roles in metabolism-released neurotransmitters [73]. Both isoforms MAO-A and MAO-B possess 73% sequence similarity; however, in the central nervous system, the MAO-A form is present mostly in catecholaminergic neurons, whereas the MAO-B form is mostly found in serotonergic neurons and in the glia [74]. ⫷ [1]
⫸ Monoamine oxidase (MAO; EC 1.4.3.4) is a flavin adenine dinucleotide (FAD) dependent enzyme which is mainly localized on the outer mitochondrial membrane, responsible for the oxidative deamination of monoamines, including neurotransmitters, such as norepinephrine, dopamine, and serotonin (5-hydroxytryptamine [5-HT]) [23,24]. The two isoforms of MAO exists MAO-A and MAO-B, which differ in amino acid sequence, susceptibility to specific inhibitors, substrate specificity, and tissue distribution [25]. MAO-A preferentially deaminates noradrenaline and serotonin (5-hydroxytryptamine), whereas MAO-B preferentially deaminates β -phenyl-ethylamine and benzylamine. Inside the brain, MAO-B is mainly localized in the glial cells, while MAO-A found in the extraneuronal compartment and inside the dopaminergic, serotonergic and noradrenergic nerve terminals [26]. ⫷ [30]
⫸ Monoamine oxidase (MAO) is an enzyme bound with the mitochondria that catalyzes the oxidative deamination of a range of neurotransmitters e.g., serotonin, tyramine, norepinephrine, and dopamine. This process produces (during the biochemical reaction) several harmful side compounds, including peroxides, ammonia, and aldehydes. This enzyme occurs in MAO-A and MAO-B isoforms. They show remarkable sequence similarity but differ in their substrate-inhibitor recognition sites and presence within the tissues. MAOs catalyze the oxidative deamination of several monoamines and play important roles in metabolism-released neurotransmitters [73]. Both isoforms MAO-A and MAO-B possess 73% sequence similarity; however, in the central nervous system, the MAO-A form is present mostly in catecholaminergic neurons, whereas the MAO-B form is mostly found in serotonergic neurons and in the glia [74]. ⫷ [1]
Aging is characterized by an increased susceptibility to chronic diseases. The shared feature of these age-related pathologies is the persistent activation of MAOs throughout multiple organs, which leads to altered substrate availability, oxidative stress or, the combination of both. Low monoamines bioavailability is particularly deleterious in depressive disorders. Likewise, increased uptake of norepinephrine by macrophages induces a reduction of adipocyte lipolysis during aging. Conversely, the production of toxic byproducts (aldehydes, H2O2) by MAOs induces a variety of deleterious effects among organs. They are particularly involved in neurodegenerative diseases like Parkinson’s and Alzheimer’s. They are also involved in cardio-metabolic disorders including age-related heart failure, obesity, diabetes and related consequences. MAOs-related oxidative stress has also important roles in tumorigenesis and metastasis, by inducing EMT, cell proliferation and stimulating angiogenesis.
(NSS) Quercetin ⫷⬆︎ induced Reprogramming ⫸
Multiple, natural, nutritional supplements have been have been identified as efficient, selective and reversible MAO's inhibitors, including, Quercetin and EGG. In 2006, Zhang et al. determined MAO inhibition of natural flavonoids by docking experiments. Docking methodologies revealed that the quercetin can thoroughly bind within the active site of the MAO-B (with drug score of -61.5). [M3]
By exhibiting IC 50value of 18 pM quercetin was was distinguished as a selective MAO-A inhibitor. Therein, quercetin, myricetin, and chrysin induced MAO-A inhibition activity with IC50 values of 9.93, 1.52, and 0.25 uM, respectively, whereas genistein was found to be a most effective potent inhibitor of MAO-B, with an IC50 value of 0.65 uM. [ 21] All isolated flavonoid derivatives were also observed as selective MAO-A inhibitors with the IC50 estimations of quercetin (4 uM), apigenin (2 uM), kaempferol (0.8 uM), and chrysin (1 uM). EGG was tested for MA0 inhibition. It showed a sigmoidal dose-response curve for MAO-B with IC50 of 4.74 uM, whereas I-deprenyl showed the IC50 value of 7.24 for MAO-B inhibition. [30,31] [M3]
Quercetin has been distinguished as a reversible and mixed type inhibitor (Chimenti et al., 2006; M. H. Lee et al., 2001; Prasopthum et al., 2015; Saaby et al., 2009). Quercetin was also reported as a selective MAO-A inhibitor (Saaby et al., 2009 .Inhibition of MAO by the phytochemicals seems to be dependent on the presence of a phenyl or hydroxypheny| ring. Rationalized docking studies enumerated the inhibitory properties of quercetin, myricetin, genistein, and chrysin against the isoforms of hMAO-A and hMAO-B. Quercetin and myricetin both interacted with Ala111, Ile180, Asn181, Thr336, and Phe208 amino acids of MAO-A. Additionally quercetin was found to bind to extra amino acids Gln215, Thr336, and Tyr444 of MAO-A.
(NSS) Curcumin ⫷⬆︎Induced Reprogramming ⫸
(NSS) Curcumin Depletion of dopamine and 3,4-dihydroxy phenyl acetic acid occurs with increased MAO-B activity. Use of curcumin 130 [178] and tetrahydrocurcumin 131 reversed the decrease in dopamine and 3,4-dihydroxy phenyl acetic acid induced by the model [179]. Curcumin: TGF-b signaling isinitiated by TGF-b binding to its cell membrane receptor and formation of the activated receptor complex, which then phosphorylates intracellular messenger molecules like SMAD proteins [2]. Our data show that curcumin decreased TGF-b receptor Il protein levels. This was associated with reduced SMAD2/3 phosphorylation after curcumin treatment, indicating reduced biological activity of the activated TGF-b receptor complex. [T16]
Mechanistically, curcumin treatment downregulated the expression of MAO-A and suppressed the STAT6 pathway. [C8]
(???) Cysteine-dependent dithiol-disulfide isomerase (CDGSH)
CDGSH stands for Cysteine-dependent dithiol-disulfide isomerase. It is a type of enzyme that acts as an oxidative stress sensor and regulator in the cell. CDGSH is involved in the maintenance of redox homeostasis, which is the balance between pro-oxidants and antioxidants in the cell, and has been implicated in a number of cellular processes including cellular signaling, protein folding, and immune response.
The expression of CDGSH is regulated by various factors and can be altered in response to various stressors, including oxidative stress. Alterations in CDGSH expression have been associated with aging and various age-related diseases. As a result, CDGSH has emerged as a potential target for interventions aimed at combating aging and age-related diseases. {CGPT3[R20]}
(???) Iron-sulfur domain 2 (CISD2)
Iron-sulfur domain 2 (CISD2) is a protein domain found in some enzymes involved in cellular metabolism. The iron-sulfur (Fe-S) cluster, a coordination complex of iron and sulfur atoms, is an essential cofactor for many enzymes and is involved in a wide range of biological processes, including electron transfer, catalysis, and regulation of gene expression.
CISD2 is a subunit of the CISD family of proteins, which are involved in the maturation of Fe-S clusters in cells. CISD2 is thought to play a role in the formation and stability of Fe-S clusters and in their transfer to target proteins. Mutations in the CISD2 gene have been associated with several human diseases, including neurodegenerative disorders and cancer, suggesting that CISD2 and the Fe-S clusters it helps to produce play an important role in human health. {CGPT3[R20]}
(???) DNA methylation
DNA methylation is a process of adding a methyl group (-CH3) to a cytosine base in DNA. DNA methylation is an epigenetic modification, meaning it affects gene expression without changing the underlying DNA sequence. DNA methylation occurs primarily on cytosine residues in CpG dinucleotides, where a methyl group is added to the carbon 5 position of the cytosine ring.
DNA methylation has a variety of biological functions, including regulation of gene expression, DNA repair, and maintenance of chromosome stability. In particular, DNA methylation is involved in the regulation of gene expression by inhibiting the binding of transcription factors and other regulatory proteins to the DNA.
DNA methylation patterns change over time and with exposure to different environmental factors, such as aging and disease. Aberrant DNA methylation patterns have been associated with a number of age-related diseases, including cancer, neurological disorders, and cardiovascular disease. Therefore, understanding the role of DNA methylation in aging and age-related diseases has important implications for developing new therapeutic strategies to prevent or treat these conditions. {CGPT3[R20]}
(???) Mitochondrial decay
Mitochondrial decay refers to the gradual decline in the function of mitochondria, the cellular organelles responsible for producing the majority of a cell's energy in the form of ATP. Mitochondria play a critical role in cellular metabolism, and the decline in their function is associated with a wide range of age-related diseases and conditions, including neurodegeneration, cardiovascular disease, and type 2 diabetes.
Mitochondrial decay is thought to result from a combination of factors, including oxidative stress, accumulation of damaged proteins and lipids, and decreased capacity to repair and replace damaged mitochondria. Over time, these processes lead to a decline in mitochondrial function, including decreased energy production and increased production of reactive oxygen species.
Mitochondrial decay is also thought to play a role in aging itself, as the decline in mitochondrial function is often accompanied by an increase in cellular and tissue damage. Understanding the mechanisms of mitochondrial decay and developing therapeutic strategies to prevent or reverse it are important areas of research for developing new treatments for age-related diseases and for extending healthy lifespan. {CGPT3[R20]}
(???) Exosomal miRNAs
Exosomal miRNAs are microRNAs that are packaged in small vesicles called exosomes and secreted from cells into the extracellular environment. miRNAs are short non-coding RNA molecules that play important roles in regulating gene expression and cellular processes.
Exosomal miRNAs are emerging as a new type of intercellular communication mechanism, as they can transfer genetic information from one cell to another through the release of exosomes. This transfer of miRNAs can have a profound effect on recipient cells by altering their gene expression patterns, leading to changes in cellular function and behavior.
Studies have shown that exosomal miRNAs are involved in a wide range of biological processes, including development, inflammation, and disease. For example, they have been implicated in the regulation of tumor progression, the spread of cancer cells, and the regulation of immune responses.
Exosomal miRNAs are also being investigated as potential biomarkers for a variety of diseases, as changes in their levels and patterns of secretion have been observed in many disease states. Understanding the role of exosomal miRNAs in disease and normal cellular processes is an important area of research with implications for the development of new diagnostic and therapeutic approaches. {CGPT3[R20]}
Multiple concomitant agents have been identified in the literature as having additive and/or synergistic benefits to effectively inducing reprogramming.
◉ (NSS) Lithium ⫷⬆︎ induced Reprogramming ⫸
(T) Natural Killer Cells (NK)
⫷⬇︎ induced Reprogramming ⫸
Click [√] to Enlarge Source: [NK4]
NK cells significantly limit reprogramming. Cells in the process of reprogramming upregulate the expression of NK-activating ligands, such as Mult1, Icam1 and Cd155. NK cells recognize and kill cells undergoing reprogramming in a degranulation-dependent manner that is partially blocked by antibodies against the NK- activating receptor NKG2D. In mice, transient depletion of NK cells using antibodies significantly improves the efficiency of reprogramming, as determined by the emergence of dysplastic cells and NANOG+ cells. Finally, organoids derived from NK-depleted reprogrammed pancreata are remarkably large, suggesting that NK cells preferentially target those cells with high organoid-formation capacity. Other cell types from the innate immune system also contribute to the modulation of in vivo reprogramming efficiency. [49] In the present study, we report that NK cells recognize and kill cells undergoing partial reprogramming. Furthermore, our results suggest that NK cells preferentially eliminate cells endowed with higher plasticity and regenerative capacity.Having established that NK activity was not significantly affected by OSKM expression in our mouse model, we then addressed the effect of eliminating NK cells on partial reprogramming. Based on these results, we propose that NK cells hinder the process of in vivo reprogramming by eliminating emerging partially reprogrammed cells. To further strengthen this hypothesis, we tested whether the adoptive transfer of exogenous NK cells reduced the normal levels of reprogramming in the pancreas. Thus, NK cells from WI donor mice were injected on day 3 of reprogramming. Importantly, exogenous transfer of NK cells dramatically reduced the efficiency of reprogramming, and no NANOG + cells were detected (Fig. 4C).[NK3] (NSS)
(D) Glucocorticoids
⫸ Glucocorticoids regulate natural killer cell function epigenetically. The administration of GC 1 hour after endotoxin challenge resulted in suppression of the proinflammatory response [51]. NK cells and Gs exert essentially the opposite effect on diseases in which the immune system is involved such as viral infections, cancer and inflammation, while in autoimmune diseases the role of NK cells can be ambivalent. These opposite effects are due, at least in part, to a direct action of inhibition of NK cells by GCs. ⫷ [✷ NK2]
(H) Oxytocin
⫸ Oxytocin is a component of the hypothalamic-pituitary axis. It is a neurohormone produced in the hypothalamus and secreted from the posterior pituitary gland and it is of vital importance to the immune and neuroendocrine systems [2]. Indeed, it has been shown that oxytocin can suppress the up-regulation of toll-like receptor 4 (TLR4) [3]; suppress the release of interleukin 6 (IL-6) [4,5], tumor necrosis factor alpha (TNF-alpha) [5]; inhibit the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [6]; increase FOXP3+ regulatory T cells [7], modulate neutrophils [8], mast cells [9] and macrophages [10]. In other words, it may be able to turn off an activated proinflammatory immune system and therefore reduce the likelihood of autoimmune disease [11]. ⫷ [10]
⫸ Here we report that oxytocin—a hormone best known for its role in lactation, parturition and social behaviours—is required for proper muscle tissue regeneration and homeostasis, and that plasma levels of oxytocin decline with age. systemic administration of oxytocin rapidly improves muscle regeneration by enhancing aged muscle stem cell activation/proliferation through activation of the MAPK/ERK signalling pathway.
Plasma levels of Oxytocin (OT) and the levels of Oxytocin receptors (OTR) in muscle stem cells dramatically decline with age and demonstrate that OT is required for skeletal muscle tissue regeneration and homeostatic maintenance. Importantly, we show that short-term systemic OT delivery restores muscle regeneration in old mice by improving aged muscle stem cell function, while pharmacologic attenuation of OT signalling with a selective antagonist alters muscle regeneration in young mice.
Oxytocin improves myogenic progenitor cell proliferation via activation of the MAPK/ERK pathway.
Our results demonstrate that OT is one of the key age-specific systemic regulators of muscle maintenance and repair; however, it is unlikely that only one circulating molecule accounts for systemic ageing or rejuvenation and other cell fate regulatory pathways have been shown to participate in these phenomena 5,24 . While other strategies such as long-term activation of the Notch signalling pathway or downregulation of the TGF-b/pSmad and Wnt signalling pathways have been shown to be successful in acute rejuvenation of myogenesis 4,5,24
OT is a naturally produced endocrine peptide that has little to no known detrimental side effects. Diminished circulating levels of OT are associated with pathological states such as autism in children 44,45 , osteoporosis 46 and depression 47 . OT is approved for use in women during parturition has been tested to improve psychological well-being in the elderly 48 and is in clinical trials to treat autism, schizophrenia and depression. Owing to its size and structure, OT can be administered easily and by multiple routes such as by intranasal inhalation. The potent positive effects of OT on muscle tissue homeostasis and repair that were uncovered in this study are thus promising for developing an effective and safe new clinical strategy where OT and OTR agonists might be potentially used as systemically applicable and sustainable molecules for combating the deterioration of muscle mass, strength and agility in the elderly. ⫷ [O1]
Oxytocin acts through the TGF- β signaling pathway to induce epicardial activation
To obtain additional clues related to the potential mechanism of action driving the effects of oxytocin, we performed RNA-seq on hEpiCs in control and OXT-treated conditions as described above. RNA was collected for transcriptomic differential gene expression analysis after 3 days of 100 nM OXT exposure. We found that oxytocin induced significant widespread gene expression changes (Figure 4A). Computational analysis using gene ontology identified upregulated and downregulated clusters consistent with our previous observations on the effects of oxytocin (induction of a progenitor-like state, increased proliferation, EMT) (Figure 4B,C). Of particular interest was the upregulation of TGF- β /BMP pathway biological processes, as well as developmental ones (Figure 4B). Among downregulated processes, a series of metabolic functions well-ascribed to the epicardium were identified, consistent with our proposed model of mature epicardial cells becoming epicardial progenitors upon OXT treatment. Leading genes driving the TGF- β pathway activation were ligands LEFTY2, GDF15, and INHBB, and the BMP pathway activator TMEM100 (Figure 4D). Overall, these data suggest that oxytocin, through the activation of OXTR, can promote the expression of proteins involved in TGF- β and BMP signaling in the epicardium, leading to increased epicardial progenitor cell mobilization. [O9]

Two Compound (2C) Reprogramming -
Overlapping Activity by Nutritional Supplements
Click [√] to Enlarge
Click {√} to Enlarge
Natural Inhibitors of Transforming Growth Factor beta-1
Natural Inhibitors of Monamir Oxidase (MAO)
Structures with cross activity are indicated with color highlights from title of that cross activity. It should be noted that there may be many other molecules that also have dual activity, but have not been as yet identified. Originally 2-factor, small molecules identified by Ocampo’s group are highlighted in gold.
ADDATIONAL MAO INACTIVATORS (IRREVERSABLE) AND REVERSIBLE INHIBITORS
Click [√] to Enlarge

DRAFT PROTOCOL
The first draft of a protocol is available by request. Please use the links at the bottom of this page to request a copy. What fellows are the key elements of the proposed study.
For individuals the cost of self monitoring can prevent them from effectively collecting enough data to make safe and effective decisions on the therapeutic benefits of any interventional protocol they are attempting to implement. We have attempted to locate Biometric, Clinical and Laboratory markers that are both cost and efficaciously effective biological markers of aging. The “Correlated Biomarkers of Aging,” Pyramid stratifies the relative cost and strength of correlation for each group of markers. Each level is linked to the individual detail page below.
Click [√] to Enlarge

PROTOCOL OVERVIEW & GOALS
Use Red Arrows to Open each Protocol Segment
-
• The primary goal of this proposed study is to determine if nutritional supplements can be safely and effective replacements for the small molecules and drugs that have been identified in recent published research.
• Safety and Tolerability [Time Frame: 6 months] Incidence of treatment-related adverse effects
• Epigenetic Age Baseline, 1, 3 and 6 months
-
A phase I-II clinical study to determine the safety, tolerability and effects of inhibiting two molecular pathway utilizing flavonoids, vitamins, minerals/metals, and supplements, addatively or synergistically augmented by vitamins(C, B12) and lithium and selenium.
Inclusive Age Eligible for Study: 40 Years to 80 Years, male or female, meeting inclusion and exclusion criteria.
√ Opportunity to discuss any questions with the medical staff of supporting institution and signing of Informed Consent.
-
• Safety and Tolerability [Time Frame: 6 months]
Incidence of treatment-related adverse effects
• Frailty Index
• Grip Strength, Baseline, 1, 3 and 6 months
• Epigenetic Age Baseline, 1, 3 and 6 months
• Laboratory Assays including:
- TGF beta
- Beta 2 Microgobolum
- Ablumin
- Macrophage/Lucocyte Ratio
- WBC
- T-Cell Differential
-
• Male or female volunteers
• Aged 55 to 80 years, inclusive
• All ethnicities
• Able to participate in 6-month study
• Able to provide informed consent
-
• AGE: 50 to 90 years of age
• Male or Female
• Malignancies or high risk of malignancy, as suggested by familial risk or personal medical history
• Premenopausal women
• Postmenopausal women on HRT
• IGF-1 levels < 90 ng/ml or >300 ng/ml
• Diagnosed or suspected growth hormone resistance
• Known growth hormone deficiency based on stimulation testing
• Pre-existing carpal tunnel syndrome
• Significant arthritis/arthralgia/joint swelling
• Bradycardia (<55 bpm), significant hypertension (systolic >160 mmHg, or diastolic >90 mmHg) despite treatment, serious angina, or other serious cardiovascular disease or cardiovascular disease risk factors
• Excessive skin growths (e.g., flat warts) without cryosurgical options
• BMI of 35 or greater
• PSA level above the age-adjusted normal range for reasons other than confirmed prostatitis
• Testosterone levels above the upper limit of normal
• Levels of C-reactive protein (CRP) above the upper limit of normal
• Type 1 or pre-existing Type 2 diabetes
• Uncorrected hypothyroidism
• HIV infection
• Allergy or other sensitivity to study medications
• Other unstable medical conditions
• Use of GH within the last 5 years
• Participation in a clinical research trial within 30 days prior to enrollment
• Use of chronic glucocorticoid therapy
• Unwilling to discontinue androgen supplementation if testosterone levels are above the upper limit of normal
• Ongoing treatment with carbonic anhydrase inhibitors
• Ketogenic diet, calorie-restricted diet, or prolonged fasting, without willingness to discontinue these diets or adhere to an alternative diet during the study
• Alcoholism or drug addiction
• Smoking or unwillingness to quit smoking
• Cognitive impairment, illiteracy, inability or unwillingness to give voluntary informed consent
-
1) Height,
2) Weight,
3) Temperature,
4) Blood Pressure
5) Measurement around waist

CLINICAL AND LABRATORY ASSESMENTS
-
1) Activities of Daily Living
1.1) A Self Evaluation of Your Quality of Life
-
Links to individual biomarkers descriptions:
(2.0) BIOMETRIC BIOMARKERS
(2.1) GRIP STRENGTH
(2.2) STEP COUNTERS
(2.3) FACIAL AGE SCANS
-
(3.1) CLINICAL BIOMARKERS
-
LAB MARKERS NORMAL RANGE
WBC 3.4-10.6 K/mm3
RBC 3.8-5.4 M/mm3
Hemoglobin 12.9-16.1 g/dL
Hematocrit 38.0-47.0%
MCV 80-100 Fl
MCH 27.0-33.0 pg
MCHC 32-37.5 g/dI
RDW 11.5-15.8%
Platelets 150-450 K/mm3
Neutrophils 36-75.2%
Lymphocytes 20.5-51.1%
Monocytes 1-9.3%
Eosinophils. 0.9-6.0%
Basophils. 0.3-1.5%
Sodium 136-145 mmol/I
Potassium 3.3-5.1 mmol/L
Chloride 98-107 mmol/L
CO2 21-32 mmol/L
Glucose 74-106 mg/dL
Urea Nitrogen 9-23 mg/dL
Creatinine 0.70-1.30 mg/dL
Calcium 8.7-10.4 mg/dl
Total Protein 5.7-8.2 g/dL
Albumin 3.2-4.8 g/dL
Total Bilirubin 0.3-1.2 mg/dL
Alkaline Phosphatase 46-116 U/L
Aspartate Aminot. (AST) <34 U/L
Alanine Aminotrans (ALT) 10-49 U/L
eGFR AF AMER ≥60 mL/min/1.73m?
eGFR NON-AF AMER ≥60 mL/min/1.73m?
Anion Gap. 5.0-15.0 mEq/L
TNF-a. 0.56-1.40 pg/ml
Interleukin-6. <2 pg/mL
d-Dimer <0.5 4g/mL FEU
C-Reactive Protein. <1.0
-
-
-
-
• CBC
• WBC’s Auto
• RBC’s Auto
• HGB
• HCT, Auto
• MCV
• MCH
• MCHC
• RDW, BLOOD
• PLATESLETS
• WBC
• Hemoglobin . HGBA1C%
• Hematocrit,,
• MCV
-
• CBC
• WBC’s Auto
• RBC’s Auto
• HGB
• HCT, Auto
• MCV
• MCH
• MCHC
• RDW, BLOOD
• PLATESLETS
• WBC
• Hemoglobin . HGBA1C%
• Hematocrit,,
• MCV
-
• WBC COUNT
• LYMPHOCYTES %
• LYMPHOCYTES COUNT,
• CD3+ T CELLS %
• CD3+ T CELLS
• CD4+CD3+ T CELLS %
• CD4+CD3+ T CELLS
• CD8+CD3+ T CELLS %
• CD8+CD3+ T CELLS
• CD4/CD8 RATIO
-
• Cholestoral
• HDL
• LDL
• TRIGLYCERIDE, FASTING
• CHOLESTEROL, NON-HDL
-
• calcium,
• phosphorus,
• potassium,
• Lithium,
• total bilirubin,
• total protein,
• albumin,
• alkaline phosphatase,
• AST (SGOT),
• ALT (SGPT),
• LDH,
• BUN,
• creatinine,
• glucose,
• uric acid,
• bicarbonate,
• cholesterol.
-
• sodium,
• potassium,
• chloride,
• CO2
-
• GLUCOSE UA
• KETONES UA,
• SPECIFIC GRAVITY, UA
• UA HGB
• PH, UA
• PROTEIN, UA
• NITRITE, UA
• LEUKOCYTE ESTERASE UA
• UA
• UROBILINOGEN, UA, QL
• BILIRUBIN, UA
• MICROSCOPIC EXAM, URINE
• PHOSPHORUS
• AST
-
• ALT
• ALKALINE PHOSPHATASE
• BILIRUBIN, TOTAL
-
• A
• B12
• C
• D
• E
-
• β 2M - Beta 2 Microgobulin
• CRP - C reactive protein
• HYC - Homocysteine
• IGF-1 - Insulin like growth factor
• IL-2 - Interleukin 2
• IL-6 - Interleukin 6
• IFNγ - Interferon gamma
• TNFα - Tumor necrosis facto alpha
• NFk β - Necular factor kappa beta
• Oxytocin
• TGF-β1 - Transforming growth factor beta 1
[1] [2020] Correlation analyses between age and indices in routine blood laboratory tests suggest potential aging biomarkers

◉Reprogrammed Induced Rejuvenation
◉ DAY 1
Epigenetic Pre-Conditioning
[2] Vit C (2000 mb BID), [1] Magnesium (1), Multi Mineral Formulation (Focus Factor)
◉ DAY 2 - 3
TGF Inhibitors:
[2] Vit C (2000 mb BID), [1] Magnesium (1), Multi Mineral Formulation (Focus Factor)
[2] Qucertine (500 mg), [2] EGCG 1 (725 mg), [2] Curcumin (500 mg), [2] Fiestein (500 mg), [2] Petrosteilbene (200 mg),
MAO Inhibitors:
*Quercetin, (MAO-A) EGCG and Curcumin (MAO-B) are dual inhibitors of TGF and MAO, and dosing information is provided above.
[1] Lithium (5 mg), Synergistic in improving inhibition with all known MAO inhibitors
Addative/Synergistic concomitant supplements
[1] Magnesium (400 mg), [1] Zinc (50 mg) [1] B-12 (5000 mcg)
E,[] D [] and Zinc [] are synergistic with Qucertine, [] Selenium is synergistic with EGCG [], Magnesium dramatically increases binding kinetics for a large number of cellular receptors. []
◉Anti-Aging Longevity associated
◉ DAY 4 & 6
[1] AKG (1,000 mg), [2], NMN (250 mg)
◉ DAY 5 & 7
[1] Glycine (), [1] NAC (), [1] Gluthaithone ()
◉ Last Revision: 11/18/22
Dosing Schedule / Rational / Safety Profile
Belmonte et al. up-regulated / activated a OSKM factors cassette in mice, daily for 8 days, via doxycycline in order to determine the safety/therapeutic profile. Both body weight and survival where the primary end points.
Image Source: [2016] In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming [11]
As they observed that some mice started to die after 3 consecutive days of OSKM induction, they settled on a cyclic 2/5-day treatment regimen: 2 days of treatment followed by a 5-day break. They started treating the mice at 8 weeks of age and continued weekly treatments until death. With this protocol Belmonte et al. observed a 33-50% median lifespan increase (depending on which control group they were compared to). Even more impressive than the median lifespan increase was the fact that by the time all mice without OSKM genes died (week 22), 75% of mice treated with epigenetic rejuvenation were still alive.
Click [√] to Enlarge
They had 3 control groups: two groups of LAKI mice without the Tet-On cassette (one of which was still getting weekly doxycycline doses to make sure the drug itself does not 26 produce any life increasing effects) and one control group with the OSKM cassette (they call it ‘4F’) but without weekly activation. The treated mice not only lived longer but also were biologically younger as measured by several biomarkers, including • lower markers of senescent cells (p16Ink4a and beta-galactosidase), • lower marker of double-stranded DNA breaks (gamma-H2AX), • lower metalloprotease levels, • lower interleukin-6 levels, • lower levels of mitochondrial reactive oxygen species (‘free radicals’), • higher number of hair follicles, and from a separate study it was determined that the same treatment increases telemor lengths.
Although previous studies have indicated that expression of the Yamanaka factors in vivo can lead to cancer development or teratoma formation (Abad et al., 2013; Ohnishi et al., 2014), here, we demonstrate that tumor formation can be avoided by short-term induction of OSKM. Cyclic induction of OSKM in vivo ameliorated hallmarks of aging and extended the lifespan of a mouse model of premature aging. Additionally, short-term induction of OSKM improved the regenerative capacity of pancreas and muscle following injury in physiologically aged mice.
Together, these results show that partial in vivo reprogramming might be used to modulate aging hallmarks and significantly benefit organismal health.
Towards this goal, we monitored survival in C. elegans treated with either 2c, Repsox, or TCP at three different concentrations (50, 100, or 200 μM) alongside a vehicle control. Strikingly, we observed that 2c treatment at 50 μM was sufficient to extend C. elegans median lifespan from 19 to 27 days, corresponding to a 42.1% increase relative to vehicle control (Fig. 4a, e). To a lesser extent, Repsox or TCP alone at 50 μM also increased C. elegans median lifespan to 25 days, a 31.6% increase over vehicle control (Fig. 4a, e). These results indicate that Repsox and TCP are each able to extend median lifespan in C. elegans, and when combined as part of the 2c cocktail, can lead to an even greater increase in median lifespan. Interestingly, the 2c cocktail or Repsox alone did not increase C. elegans lifespan at 200 μM (Fig. 4b-c, e), suggesting that this higher dose may impact off target mechanisms and be slightly toxic. On the other hand, TCP still increased median lifespan by 15.8% at 100 or 200 μM relative to vehicle controls even though it was most effective at 50 μM (Fig. 4d-e). Taken together, these data demonstrate that the optimized 2c cocktail can both ameliorate multiple aging hallmarks in aged human fibroblasts in vitro and extend C. elegans lifespan in vivo.
Click [√] to Enlarge
Figure 4 (above) | Treatment with 2c increases C. elegans lifespan.
Survival of N2 C. elegans upon treatment of TCP (50 µM), Repsox (50 µM), and 2c (TCP + Repsox, 50 µM each). b-d, Survival of N2 C. elegans upon treatment with 2c (b), Repsox (c), and TCP (d) at 50, 100, or 200 µM. e, Summary of survival assay results including median lifespan, maximal (90%) lifespan, and statistical analyses. Median lifespan increase relative to vehicle control. Statistical significance was assessed by comparison to untreated control using Log-Rank (Mantel-Cox) test.

🔷 ? 🔷

Epigenetic of reprogramming induced rejuvenation. Aging is characterized by a gradual loss of function occurring at the molecular, cellular, tissue and organismal levels. At the chromatin level, aging associates with progressive accumulation of epigenetic errors that eventually lead to aberrant gene regulation, stem cell exhaustion, senescence, and deregulated cell/tissue homeostasis. Notably, if the expression of the reprogramming factors is only transiently applied and then stopped (before the so-called Point of No Return, PNR), the cells return to the initiating somatic cell state. These observations suggest that, if applied for a short enough time, the expression of reprogramming factors fails to erase the epigenetic signature defining cell identity…. [✷ RIR6]

◉ SAFETY PRECAUTION:
Inhibiting the Monoamine oxidase (MAO) enzymes has the potential to cause severe, even life threatening side effects when certain foods with high thiamine content are taken at the same time. A primer on the necessary precautions is provided in a dedicated section below. Do not discount the potential risk of this because nutritional supplements are bing utilized. This potential age-regression intervention should not be undertaken without the knowledge and oversight of your primary care physician.
◉ Potentially Serious Supplement/Food Interactions
Risk of Dangerous Increases in Blood Pressure:
Certain foods and drinks must be avoided during treatment with all MAO inhibitors and for 2 weeks after stopping treatment. If you do not avoid certain foods and drinks, you may have a dangerous increase in blood pressure that can lead to death. A complete list of those foods and drinks are included below in the section called "Foods and Beverages with a High Tyramine Content.”
Click [√] to Enlarge Source: [M9]
Foods and Beverages with a High Tyramine Content: When excessive amounts of tyramine are consumed in conjunction with MAO inhibitors, or within 2 weeks of stopping administration, a serious and sometimes fatal hypertensive reaction may occur.
Meat, Poultry, and Fish (MPF): Air dried, aged and fermented meats, sausages and salamis (including cacciatore, hard salami and mortadella); pickled herring; and any spoiled or improperly stored meat, poultry, and fish (e.g., foods that have undergone changes in coloration, odor, or become moldy); spoiled or improperly stored animal livers.
Acceptable MPFs: Containing No or Little Tyramine: Fresh meat, poultry, and fish, including fresh processed meats (e.g., lunch meats, hot dogs, breakfast sausage, and cooked sliced ham).
Vegetables: Broad bean pods (fava bean pods)
Acceptable Vegtables: All other vegetables.
Dairy Products: Aged cheeses
Acceptable Dairy Products: Processed cheeses. mozzarella, ricotta cheese. cottage cheese, and yogurt.
Beverages: All varieties of tap beer and beers that have not been pasteurized so as to allow for ongoing fermentation and
excessive amounts of caffeine. Concomitant use of alcohol with MAO inhibitors is not recommended.
Acceptable Beverages: (Bottled and canned beers and wines contain little or no tyramine.)
Other: Concentrated yeast extract (e.g., Marmite), sauerkraut, most soybean products (including soy sauce and tofu), OTC supplements containing tyramine, and chocolate
Acceptable Others: Brewer's yeast, baker's yeast, soy milk, commercial chain restaurant pizzas prepared with cheeses low in tyramine.
MAOi-Herb Interactions: Caution should be exercised when giving PARNATE with St. John's wort, as the development of serotonin toxicity has been reported with MAOIs when given before, with, or shortly after discontinuation of this drug. Observation of tranylcypromine washout period of at least 1 week is recommended.
Insomnia: It is the most frequent adverse reaction to MAO inhibitors, which can usually be overcome by taking the RIR supplements before 3 PM.
Do not take MAO inhibitors of any source if you:
• are allergic to the active compounds identified in this protocol or is you have experience adverse reactions to with a mechanism of action that inhibits Monamine Oxidase such as tranylcypromine(Parnate).
• have high blood pressure or heart disease
• have ever had a stroke, or any other kind of disorder related to the brain and its blood vessels
• have liver disease
• have a blood disorder
• have a history of severe or frequent headaches
• have a tumor of the adrenal gland (pheochromocytoma) or a type of tumor called a paraganglioma
• ¡were recently or are taking any other medicine for depression or anxiety
Were or are taking any of the following:
• carbamazepine, used to treat seizures
• cyclobenzaprine, used as a muscle relaxant
• sumatriptan, rizatriptan and other triptans, used to treat migraines and cluster headaches
• non-prescription medications to treat colds or hay fever, including drops or sprays
• natural health products that are for increased energy or weight loss
• street drugs including MDMA and ecstasy
• amphetamines, used to treat attention deficit disorder
• products containing ephedrine, used to treat nasal congestion
• methyldopa, used to treat high blood pressure
• dopamine, used in hospital to treat certain medical emergencies
• levodopa, used to treat Parkinson's disease
• dextromethorphan, used as a cough suppressant
Soucre: Modified from: Parent; Product Monograph Glaxo Smith Klein
🔷 ? 🔷

🔷 ? 🔷

References are stratified by RIR molecular targets and the agents inhibiting or activating them.
Highly Relevant Papers have a Star “✷” Inserted at the Beginning of the Reference Title.
◉ REPROGRAMMING INDUCED REJUVENATION
[RIR] ◉ Reprogramming Induced Rejuvenation
[RIR1] [53] [2013] Conversion of human fibroblasts to angioblast-like progenitor cells
[RIR2] [16] [2014] Reprogramming Can Be a Transforming Experience
[RIR4] [11] [2016] [✷] In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming
[RIR5] [24] [2020] [✷] Small-molecule-mediated reprogramming: a silver lining for regenerative medicine
[RIR7] [2] [2020] Reprogramming to recover youthful epigenetic information and restore vision
[RIR8] [3] [2021] Cellular reprogramming and epigenetic rejuvenation
[RIR9] [23] [2021] Multi-omic rejuvenation of human cells by maturation phase transient reprogramming
[RIR11] [5] [2022] Aging Delayed in Mice through Longer-Term Partial Reprogramming
[RIR12] [6] [2022] Cellular Rejuvenation Therapy Safely Reverses Signs of Aging in Mice
[RIR13] [7] [2022] [✷] Chemical reprogramming ameliorates cellular hallmarks of aging and extends lifespan
[RIR14] [9] [2022] Multi-omic rejuvenation of human cells by maturation phase transient reprogramming
[RIR15] [10] [2022] Multi-omic rejuvenation of naturally aged tissues by a single cycle of transient reprogramming
[RIR16] [12] [2022] In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice
[RIR18] [15] [2022] Cellular reprogramming and the rise of rejuvenation biotech
[RIR19] [16] [2023] Loss of epigenetic information as a cause of mammalian aging
[RIR21] [2019] Mechanisms of cellular rejuvenation
◉ PRIMARY MOLECULAR TARGETS
[T] ◉ Transforming Growth Factor beta-1 ( TGF-β1 ) (⬇︎)
[T1] [7] [2006] Signal Processing in the TGF-b Superfamily Ligand-Receptor Network
[T3] [2009] Relative roles of TGF-b1 and Wnt in the systemic regulation and aging of satellite cell responses
[T5] [47] [2009] Repsox Hit a Home run
[T6] [2014] Regulation of TGF-β Signal Transduction
[T9] [6] [2017] Molecular docking analysis of curcumin analogues against kinase domain of ALK5
[T10] [48] [2019] TGF-β Signaling in Cellular Senescence and Aging-Related Pathology
[T12] [15] [2019] Epigallocatechin gallate (EGCG) suppresses epithelial-Mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways
The changes that manifest with aging include altered cell metabolism, increased Reactive Oxygen Species (ROS), inflammation, senescence, and decline in immune function. However, from the viewpoint of tissue maintenance and regeneration, we postulated that these arise from changes in tissue growth and homeostasis and specifically in key signaling networks regulating stem cells and their differentiated niches. In support of this idea, pathway modifier-based approaches for the enhancement of aged tissue repair and maintenance have been reported, for example, by systemic delivery of OT which induces MAPK/pERK signaling [12], by forced activation of Notch-1 [13], by antagonism of TGF-beta/pSmad signaling [14], or by antagonism of the Jak/Stat pathway [15].
[T14] [2019] [✷] TGFβ Family Signaling Pathways in Pluripotent and Teratocarcinoma Stem Cells’ Fate Decisions: Balancing Between Self-Renewal, Differentiation, and Cancer
[T15] [24] [2020] [✷] Small-molecule-mediated reprogramming: a silver lining for regenerative medicine
[T17] [2020] Cell Memory and Fate in Human Development Development
[T19] [n1] [2022] Controlling TGF-β signaling
[M] ◉ Monoamine Oxidase(MAO) (⬇︎)
[M2] [27] [2006] Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications
[M3] [32] [2006] Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer's disease
[M5] [2008] Monoamine oxidase inactivation: from pathophysiology to therapeutics
[M6] [29] [2008] Relationship of neurotransmitters to the symptoms of major depressive disorder.
[M7] [31] [2009] Monoamine oxidase inhibition by Rhodola roses roots
[M8] [2009] MAO-A inhibitory activity of quercetin from Calluna vulgaris
[M9] [2014] Monoamine Oxidase Inhibitors: A Modern Guide to an Unrequited Class of Antidepressants
[M13] [38] [2021] Monoamine oxidases in age-associated diseases- new perspectives for old enzymes
[M14] [20] [2022] MAOI Antidepressants- Could They Be a Next-Generation ICB Therapy
[M18] [2022] Deletion of monoamine oxidase A in a prostate cancer model enhances anti-tumor immunity through reduced immune suppression
[M19] [2022] Promising botanical-derived monoamine oxidase (MAO) inhibitors: pharmacological aspects and structure-activity studies
[H] ◉ Histone Deactylase
[GS] ◉ GSK-3b
[IL] ◉ IL-6
[N] ◉ Nanog (⬆︎)
[2022] Chapter 11 - Nanog in iPS cells and during reprogramming∗
The establishment of the very first induced pluripotent stem cells was a milestone that started a new era with fascinating potential applications in regenerative medicine and disease modeling. Surprisingly, the transcription factor Nanog was not required for this achievement, although its central role in the regulatory network governs pluripotency during embryo development and in embryonic stem cells. Soon after, it emerged again revealing its crucial role during reprogramming. Mainly, Nanog rewires the transcriptional networks promoting self-renewal and blocking differentiation, both by fine-tuning epigenetic remodeling and by direct interaction with other transcription factors. Moreover, it has shown to be a promising oncogenic biomarker and therapeutic target, linking the research fields of stem cells and cancer. Focusing on the central question of how terminally differentiated cells go back to reach pluripotency through the reprogramming process, in this chapter we review outstanding studies that evidence the crucial role of Nanog in ground state pluripotency acquisition during reprogramming and the mechanisms involved in its function.
[PD] ◉ PDK1
[NK] ◉ Natural Killer Cells (⬇︎)
[NK1] [51] [2014] Glucocorticoids regulate natural killer cell function epigenetically
[NK3] [50] [2022] Natural killer cells act as an extrinsic barrier for in vivo reprogramming
[NK4] [52] [2022] Glucocorticoids and natural killer cells: A suppressive relationship
[NO] ◉ Notch
[NO1] [2003] Notch-Mediated Restoration of Regenerative Potential to Aged Muscle
[W] ◉ Wingless (Wnt) / Canacin
Wnt signaling is functionally age dependent; promoting myogenic lineage progression ▲ during development and downregulate ▼ myogenic fate of muscles with age, instead, facilitating satellite cell conversion to a fibrogenic fate. [ 16 , 17 , 18 , 19 , 20 , 21 ]
A few pro-ageing circulating factors that increase in old animals have been identified, including transforming growth factor (TGF)-β and Wnt signalling pathway effectors, which are deleterious for muscle regeneration5,10, as well as the CCL11 chemokine that leads to impaired neurogenesis and decreased cognition and memory6
[W2] [2007] Increased Wnt Signaling During Aging Alters Muscle Stem Cell Fate and Increases Fibrosis
[W3] [2007] Wnt, the Fountain of Youth?
[W4] [2007] Augmented Wnt Signaling in a Mammalian Model of Accelerated Aging
[W6] [2011] Physiological inhibitors of Wnt signaling
◉ SMALL MOLECULES
[P] ◉ Parnate
[P2] [7] [2022] ✷ Chemical reprogramming ameliorates cellular hallmarks of aging and extends lifespan
[RS] ◉ RepSox
[RS1] [47] [2009] Repsox Hit a Home run
[RS3] [7] [2022] ✷ Chemical reprogramming ameliorates cellular hallmarks of aging and extends lifespan
[G] ◉ Glucocorticoids
[G1] [51] [2014] Glucocorticoids regulate natural killer cell function epigenetically
[G2] [52] [2022] Glucocorticoids and natural killer cells: A suppressive relationship
◉ NUTRATIONAL SUPPLEMENTS
[C] ◉ Curcumin
[C1] [2008] Antidepressant activity of curcumin: involvement of serotonin and dopamine system
[C4] [6] [2017] Molecular docking analysis of curcumin analogues against kinase domain of ALK5
[C5] [2017] Molecular docking analysis of curcumin analogues against kinase domain of ALK5
[C6] [2019] The Role of Curcumin in the Modulation of Ageing
[C7] [18] [2022] The role of dietary polyphenols in alternating DNA methylation in cancerg
[E] ◉ Ellagic Acid Galloflavin, (EGCG)
[E4] [15] [2019] Epigallocatechin gallate (EGCG) suppresses epithelial-Mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways
[F] ◉ Fisetin
[F1] [2019] New Perspectives for Fisetin
[L] ◉ Lithium
Results: In humans, we find here an inverse correlation between drinking water lithium concentrations and all-cause mortality in 18 neighboring Japanese municipalities with a total of 1,206,174 individuals (β = -0.661, p = 0.003). Consistently, we find that exposure to a comparably low concentration of lithium chloride extends life span of C. elegans (p = 0.047).
Conclusions: Taken together, these findings indicate that long-term low-dose exposure to lithium may exert anti-aging capabilities and unambiguously decreases mortality in evolutionary distinct species. [95]
Ref links to be incorporated: https://novoslabs.com/novos-anti-aging-longevity-supplement/lithium-anti-aging-supplement-longevity/
[L2] [36] [2011] ✷ Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells
[L3] [2011] Low-dose lithium uptake promotes longevity in humans and metazoans
[Q] ◉ Quercetin
[Q3] [2009] MAO-A inhibitory activity of quercetin from Calluna vulgaris
[Q5] [71] [2020] SIRT1 Activation by Natural Phytochemicals: An Overview
[Q7] [18] [2022] The role of dietary polyphenols in alternating DNA methylation in cancer]
[Q8] [2022] Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2
[R] ◉ Resveratrol
Resveratrol treatment appears to increase expression and deacetylation of PGC-1 leading to significantly elevated mitochondrial DNA and content which appears to function in part through AMPK activation and through increased SIRT expression. Supplementation with resveratrol has been shown to improve many clinically meaningful characteristics including circulating lipids, glucose, and inflammatory markers. Despite some controversy regarding the exact mechanism(s) of action, resveratrol appears to be a potent stimulator of PGC-1 and mitochondrial biogenesis in skeletal muscle. [R5]
[R2] [2018] Biological Activities of Stilbenoids
[R5] [71] [2020] SIRT1 Activation by Natural Phytochemicals: An Overview
[R8] [18] [2022] The role of dietary polyphenols in alternating DNA methylation in cancer
[R10] [2021] Targeting cancer stem cells by nutraceuticals for cancer therapy.
[S] ◉ Selenium
[S2] [2020] Association of dietary selenium intake with telomere length in middle-aged and older adults
◉ INDUCED PLURIPOTENT STEM CELLS
[IP] ◉ Induce Pluripotent Stem Cells (iPSC)
[IP3] [19] [2018] Human pluripotent reprogramming with CRISPR activators
[IP4] [18] [2019] Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications
◉ HORMONES
[O] ◉ Oxytocin
[O1] [2014] Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration
[O3] [2018] The Oxytocin Receptor: From Intacellular Signaling to Behavior
[O4] [2019] Rejuvenating Strategies of Tissue-specific Stem Cells for Healthy Aging
[O6] [2020] An Allostatic Theory of Oxytocin
[O8] [2022] Role of circulating molecules in age-related cardiovascular and metabolic disorders
[O9] [2022] Oxytocin promotes epicardial cell activation and heart regeneration after cardiac injury
[10] [2021] Could Oxytocin reduce autoimmune disease in COVID-19
◉ VITAMINS
[VB] ◉ Vit B12 (⬆︎)
[VC] ◉ Vit C (⬆︎)
[VC2] [2010] Vitamin C enhances the generation of mouse and human induced pluripotent stem cells.
[VC3] [2018] Vitamin C in Stem Cell Reprogramming and Cancer
[VC4] [40] [2019] Reprogramming the Epigenome With Vitamin C
[VC6] [2021] [✷] Ascorbic Acid in Epigenetic Reprogramming
[VD] ◉ Vitamin D3 (⬆︎)
[VD1] [54] [2014] Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism
[VD3] [55] [2020] The Relationship Between Vitamin D and Telomere/Telomerase: A Comprehensive Review
[VD4] [42] [2022] Vitamin D supplementation is associated with slower epigenetic aging